- Poster by: Adelina Tan
- Presented at: National Medicines Symposium
- Presentation date: 19-20 May 2016
- Poster summary: The Medicine Shortages Information Initiative (MSII) website is a tool for doctors and consumers to keep abreast of the current supply status of prescription medicines. You can subscribe to the Medicines Shortages email list to receive notifications when a new or updated medicine shortage is reported by sponsors to the TGA and published on the MSII webpage.
Poster: Medicine Shortages Information Initiative
Ms Adelina Tan, Mr Rob Crowdy, Ms Jocelyn McDonald
Supporting decision making for health professionals and consumers
Background
- Temporary shortages of medicines are not uncommon.
- Causes include:
- disruption to manufacturing processes
- unavailability of raw materials
- manufacturing difficulties
- changes in product formulation or manufacturer
- commercial decisions by sponsors
- industry consolidation
- unexpected increases in demand due to disease outbreak or new clinical practice.
- It is usual practice for the TGA to work with industry to minimise any disruption to the availability of medicines.
- The Medicine Shortages Information Initiative (MSII) website was launched on 26 May 2014.
- The MSII provides information, mostly based on information provided voluntarily by sponsors, about prescription medicine shortages in Australia.
- The MSII website, hosted by the TGA, is the key tool for delivering consolidated information to support health professionals and consumers when there is a temporary or permanent disruption to the supply of a medicine in Australia.
Communication and publication of information on medicine shortages
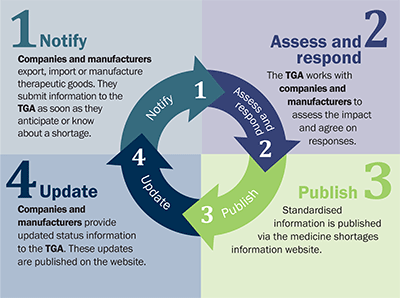
1. Notify
Companies and manufacturers export, import or manufacture therapeutic goods. They submit information to the TGA as soon as they anticipate or know about a shortage.
2. Assess and respond
The TGA works with companies and manufacturers to assess the impact and agree on responses.
3. Publish
Standardised information is published via the medicine shortages information website.
4. Update
Companies and manufacturers provide updated status information to the TGA. These updates are published on the website.
Statistics for 2015
A total of 203 temporary medicine shortages notifications were submitted to the TGA which included 40 anticipated shortages. Of the 203 notifications, 100 were related to the allergen product shortages. The emaining 103 were for other prescription medicines.
Reasons for shortages
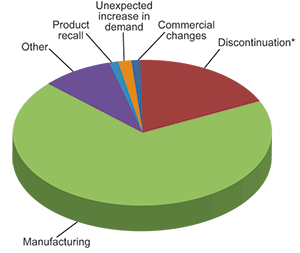
Manufacturing – 171
Discontinuation (*permanent medicine shortage) – 45
Other – 22
Unexpected increase in demand – 4
Commercial changes – 3
Product recall – 3
Notifications by Anatomical Therapeutic Chemical code
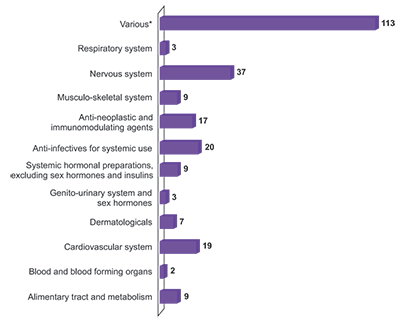
Various* - 113
Respiratory system - 3
Nervous system - 37
Musculo-skeletal system - 9
Anti-neoplastic and immunomodulating agents - 17
Anti-infectives for systemic use - 20
Systemic hormonal preparations, excluding sex hormones and insulins - 9
Genito-urinary system and sex hormones - 3
Dermatologicals - 7
Cardiovascular system - 19
Blood and blood forming organs - 2
Alimentary tract and metabolism - 9
* Includes allergen products
What can you do?
- Access MSII from the TGA homepage (www.tga.gov.au) by clicking 'Medicine shortages' under the 'Search databases' on the right side of the page.
- Subscribe to the Medicine Shortages email list or RSS feed by clicking on 'Subscribe to updates' in the 'Related information' box on the right of the page. Subscribers receive a notification when a new or updated medicine shortage is reported by sponsors to the TGA and published on the MSII webpage.
Medicine Shortages Information Initiative
| Shortages support and advice | Contact TGA |
|---|---|
|
www.tga.gov.au/medicine-shortages-information-init... 02 6232 8101 |
1800 020 653 |



