Medical Devices Safety Update is the medical device safety bulletin of the Therapeutic Goods Administration (TGA)
In this issue
Preventable alarm issues remain a major source of adverse event reports
Failure to generate alarms, inaudible alarms, or failure to trigger alarms continue to be a major subjects of adverse event reports received by the TGA.
One Australian hospital, in consultation with the TGA, recently undertook a review into its use of cardiotocographs (CTGs) following the death of a foetus.
The review found that a number of these devices in use at the facility had their alarms disabled.
A TGA investigation noted that a number of CTG devices on the market could have their default alarm settings to 'off' or 'disabled'. This would mean that if a situation warranting clinical attention developed, no clinical alarms would be generated to warn the clinician.
The TGA is continuing to investigate this issue and encourages hospitals and health professionals to review and test alarm generation capability and settings of all devices monitoring life-critical function.
Advice for health workers and facilities
Health workers and facilities should:
- regularly test alarms on devices that monitor critical physiological parameters
- review the alarm settings of devices periodically to ensure they are correct and suit your needs
- review training and educational opportunities available to staff regarding alarms
- increase staff awareness of the implications of muting alarms and changing alarm settings
- avoid exclusive reliance on audio alarms
- be aware that some manufacturers have factory default alarms set to 'off' or 'technical alarms only' – these could cause a risk to users and patients if not reviewed
- be aware that issues can happen due to a defect with the device, especially with the speakers, an incorrect alarm setting, users muting the device or setting it off accidentally
- report any missed alarms or adverse events to the TGA.
Advice for manufacturers and sponsors
Manufacturers and sponsors should:
- check that the default settings of their devices are designed and tested from the perspective of safety and that it needs to conform to Essential Principles
- review the ability of users to mute alarms permanently
- report alarm-related issues to TGA
- ensure their post-market process appropriately trigger corrective and preventative action and correct any issues early
- ensure corrective action and recalls address all devices, not just newly manufactured units.
Review into cochlear implants and magnetic resonance imaging safety
The TGA has received a number of adverse event reports relating to cochlear implants and magnetic resonance imaging (MRI) procedures.
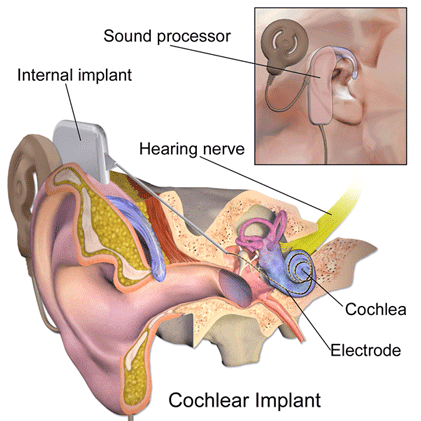
Image created by Blausen.com staff and published under Creative Commons licence.
"Medical gallery of Blausen Medical 2014". WikiJournal of Medicine 1 (2).
DOI:10.15347/wjm/2014.010
ISSN 2002-4436. Own work, CC BY 3.0.
Cochlear implants are small electronic devices that are used to stimulate the auditory nerve to provide a sense of sound to someone with profound hearing loss.
A cochlear implant has multiple parts including external parts consisting of a microphone and auditory processor as well as a transmitter. Other parts of the implant are located under the skin, including the receiver. The electrode or electrode array is inserted into the cochlea and stimulates the auditory (hearing) nerve. Magnets in both the internal and external parts of the implant allow them to connect and remain in place.
The TGA had received 18 Device Incident Reports relating to MRI safety and cochlear implants as of 9 March 2018. These reports concerned issues relating to the device's internal magnet dislodgement and reversal, subsequent surgeries to replace magnets, and pain and discomfort experienced by patients undergoing MRI while the internal device magnet is in situ.
TGA action
The TGA sought advice from the Advisory Committee on Medical Devices in February 2017 regarding the risks associated with MRI scans and patients with cochlear implants.
A post-market review was conducted on all cochlear implants supplied in Australia to assess the current procedures and advice related to MRI scans and cochlear implants.
The TGA is currently working with sponsors of cochlear Implants to update the Instructions for Use and associated product materials. The materials provided with the cochlear implant should address and advise users and clinicians about the risks when having an MRI scan with a cochlear implant.
Any decision to authorise an MRI scan remains a medical decision balancing the risk of damage to the implant and possible pain and discomfort against the benefit of information provided by the scan.
NHS cylinder alert
The United Kingdom's National Health Service (NHS) has issued a safety alert about risks associated with newer designs of oxygen cylinders.
The TGA has received reports relating to fire, leakage and broken valves and regulators but none for the reported UK issues.
The NHS said insights from local investigations prompted the following advice for facilities to focus on: prioritising training for staff groups and clinical areas where the risk is high; reinforcing theoretical training with regular opportunities to practise operating the cylinder controls; linking safe operation of cylinder controls with other key safety issues, including fire hazards and how long a full cylinder will last; and placing laminated guides close to the point of use.
Recent safety alerts
Below are TGA safety alerts relating to medical devices published since the last edition of Medical Devices Safety Update.
Rite Aid Mini Digital Temple Touch Thermometer: Recall - potential risk of harm for children who access the button battery
Puritan Bennett 980 Series Ventilator: New suspension – Medtronic Puritan Bennett 980 Series Ventilator
Therakos Cellex Photopheresis System: Update: Therakos Cellex Photopheresis System Safety advisory – risk of blood clots
TGA actions after review into urogynaecological surgical mesh implants: Update – Stress Urinary Incontinence (SUI) mid-urethral slings
 What to report? Please report adverse events, as well as near misses
What to report? Please report adverse events, as well as near misses
The TGA encourages the reporting of any suspected adverse event or potential adverse event relating to a medical device. Adverse events can involve actual harm to a patient or caregiver, or a near miss that may have resulted in harm.
Some issues relating to medical devices that may lead to adverse events and prompt you to report include:
- mechanical or material failure
- design issues
- labelling, packaging or manufacturing deficiencies
- software deficiencies
- device interactions
- user/systemic errors.
Suspected adverse events or near misses can be reported directly to the TGA:
- online at Report a problem
- by emailing iris@tga.gov.au
- by mail to IRIS, TGA, PO Box 100, Woden ACT 2606
- by fax to 02 6203 1713.
For more information about reporting, visit www.tga.gov.au or contact the TGA's Medical Devices Branch on 1800 809 361.
Disclaimer
The Medical Devices Safety Update (MDSU) is aimed at health professionals and is intended to provide practical information on medical device safety, including emerging safety issues. The information in the MDSU is necessarily general and is not intended to be a substitute for a health professional's judgment in each case, taking into account the individual circumstances of their patients. Reasonable care has been taken to ensure that the information is accurate and complete at the time of publication. The Therapeutic Goods Administration gives no warranty that the information in this document is accurate or complete, and does not accept liability for any injury, loss or damage whatsoever, due to negligence or otherwise, arising from the use of or reliance on the information provided in this document.
© Commonwealth of Australia 2018
This work is copyright. You may reproduce the whole or part of this work in unaltered form for your own personal use or, if you are part of an organisation, for internal use within your organisation, but only if you or your organisation do not use the reproduction for any commercial purpose and retain this copyright notice and all disclaimer notices as part of that reproduction. Apart from rights to use as permitted by the Copyright Act 1968 or allowed by this copyright notice, all other rights are reserved and you are not allowed to reproduce the whole or any part of this work in any way (electronic or otherwise) without first being given specific written permission from the Commonwealth to do so. Requests and inquiries concerning reproduction and rights are to be sent to the TGA Copyright Officer, Therapeutic Goods Administration, PO Box 100, Woden ACT 2606 or emailed to tga.copyright@tga.gov.au.
For the latest information from the TGA, subscribe to the TGA Safety Information email list.
For correspondence or further information about Medical Devices Safety Update, contact the TGA's Medical Devices Branch at iris@tga.gov.au or 1800 809 361.
Medical Devices Safety Update is written by staff from the Medical Devices Branch.
Editor: Ms Pamela Carter
Deputy Editor: Mr Aaron Hall
TGA Chief Medical Adviser: Adjunct Professor Tim Greenaway
Contributors include: Ms Jane Shum and Mr Kelly Tsang
Supporting documents
Print version
Related content
-
Surgical staplers: TGA reviews device incident reports
Safety updatesHealth facilities and staff are reminded of the importance of following the Instructions for Use (IFU) for surgical staplers -
Consider the whole picture when testing troponin levels
Safety updatesHealth professionals should be alert to the potential for false positive results and the associated risk of unnecessary patient treatment -
Updated cleaning instructions for long-term nasogastric tubes
Safety updatesChanges to the cleaning instructions for long-term nasogastric tubes have been implemented following a TGA investigation

