Medical Devices Safety Update is the medical device safety bulletin of the Therapeutic Goods Administration (TGA)
In this issue
Case studies in Incident Report Investigations
In the next few issues the team at Medical Device Safety Update will publish a series of case studies. The aim is to highlight the value of reporting of medical device events and problems to the TGA, as the case studies demonstrate that the TGA can often contribute towards significant positive outcomes. We will also be highlighting key lessons that can be learnt from the cases and hope that readers will also find the stories interesting. The issues described are now resolved, so if you are experiencing a problem that is similar, REPORT IT!
Study No. 1: Yellowing of epidural catheters
Our first case study is about an epidural catheter. Three unopened epidural kits were forwarded to us by a nurse. The incident description in the reporting form read simply:
"These catheters are yellow. Should be clear blue. Are they safe to use?"
Incident Report Investigation Scheme (IRIS) standard procedure is to send samples to the sponsor (supplier) of the product in Australia and to ask questions about the device and the reported problem (how often the problem has been reported before, what does the manufacturer believe the root cause to be, what are the intended corrective actions). On this occasion, it was possible to send one of the affected kits to the manufacturer, keeping two. We also asked for new kits for comparison purposes.
The sponsor's reply indicated that the catheter was made of nylon and that "the catheter had been in use for 30 years without any problems". The sponsor speculated that the yellowing was the consequence of subjecting the kits to strong heat or U/V light during transportation or storage, but the IRIS investigator thought that this was not likely... as only the catheter looked yellow while the packaging and the other kit components appeared to be normal. The TGA investigator decided to have the catheter samples tested at the TGA's Laboratories.
The tensile strength and bending properties of the complaint catheters were the same as those of new catheters, and accelerated ageing under strong light and heat melted and turned the packaging brown - but the kit contents were not affected.
Discovery in the labs
The yellowed catheter recorded the highest cytotoxicity result that the TGA labs had ever witnessed, until they tested the cytotoxicity of the new, unaffected catheter material, which was even higher. The leachate that was poisoning the cytotoxicity test cell lines was later found to be n-butyl benzene sulphonamide - a plasticiser used to make nylon plastics softer and more pliable. Additional research revealed N-butyl benzene sulphonamide to be a powerful neurotoxin.
The TGA argued that the catheter had to be reformulated - as neurotoxins should not be placed into close contact with nerve tissue (see Figure 1). The argument was difficult because even though the catheter had been in use for many years there had been no reports of problems directly associated with any form of toxicity or biocompatibility. A report of our findings was shared with other regulatory agencies and they too exerted pressure for action, so the catheter (there were no others like it) was reformulated.
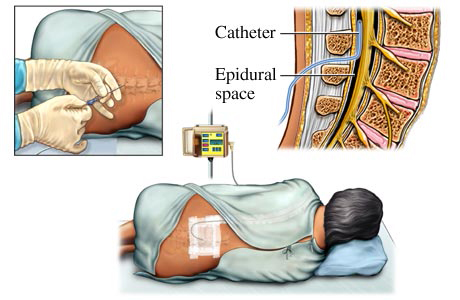
Figure 1: Placement of epidural catheters - The catheter comes into close contact with nerve tissue.
The investigation did not reveal the cause of the yellowing. It was an isolated incident most likely caused by a contaminant introduced during manufacture.
Key ideas and lessons
The TGA has extensive and well-equipped laboratories. They are used for product surveillance and can be a powerful tool when investigating incident reports. Testing is time consuming and resource intensive and so it's used only when a risk assessment indicates that its required.
As it was in this case, serendipity can be a powerful ally during an investigation, but can't always be relied upon to detect problems. Detection of issues relating to - for example - biocompatibility or the accuracy and specificity of invitro diagnostic devices require other strategies such as premarket assessment and audit, post-market review and testing.
TGA reviews reusable biopsy forceps devices
The TGA is reviewing reusable biopsy forceps devices with a focus on ensuring adequate instructions on cleaning, disinfection and sterilisation.
Any infectious agents introduced into the body can establish an infection. Medical devices or equipment that requires reprocessing - cleaning, disinfection and/or sterilisation - have the potential to spread infectious agents via ineffective cleaning procedures.
As medical devices have become more complex the issues of how to perform acceptable cleaning, disinfection and sterilisation have become greater in recent years.1
The TGA's post-market review of reusable biopsy forceps devices was undertaken following concerns being raised about one device that was being supplied in a non-sterile manner but utilised as a re-useable biopsy forcep device. Specific concerns were expressed regarding the potential risk to patients as the cleaning and sterilisation instructions and procedures were believed to be insufficient.
Reusable biopsy forceps typically have a cleaning port for flushing detergent and rinse water through their internal shaft. However, as the lumen is only open at the distal end, flushing is not entirely effective. An alternative method of flushing and then aspirating cleaning fluid through the single opening has been shown to spread contaminants throughout the device.2
Issues identified in this review include investigating whether:
- device design may not facilitate easy and effective cleaning
- Instructions for Use (IFU) and sterilising instructions are adequate
- IFU lack information regarding the number of times reprocessing of a device can occur, or visual indicators to aid in determining device deterioration and end of service life
- the risk of cross-contamination and spread of infection from a device that is not effectively reprocessed is insufficiently mitigated.
The TGA is reviewing the cleaning and disinfection/sterilisation procedures for the relevant entries on the Australian Register of Therapeutic Goods (ARTG) to determine their adequacy.
Any updates to cleaning and disinfection procedures will be provided to users of these devices in accordance with the Uniform Recall Procedures for Therapeutic Goods.
There are multiple alternative, single-use sterile devices included on the ARTG and available for use in Australia.
If you have any concerns regarding the reprocessing of a device of this kind, please contact the Australian sponsor of the affected device.
References
- Wade, W et al. 2015. Beyond Traditional Biosafety, Applied Biosafety, vol.20, No.2, 2015
- Fireman, V. 2006. Biopsy Forceps: Reusable or disposable? Journal of Gastroenterology and Hepatology 21 (7): 1089-1092
Patient lifters save staff from injuries, but care is required
While the widespread introduction of patient lifters has greatly reduced injury rates among health facility staff in recent years, the devices can pose a risk to patient safety if not used correctly.
Back injury is one of the most common causes of time off work among patient care staff and often leads to a loss of staff from the industry. Back injury is a major contributor to the ongoing shortage of nursing staff.[1]
The issue led to the introduction of 'no lift' policies in many Australian health facilities from 1998 onwards and this move has greatly reduced the rate of staff injury, time off work and worker's compensation claims.[1]
Facilities have turned to the use of patient lifters as an alternative to manual lifting and these back-saving devices are now commonly used in a wide variety of settings.
There are many models, many styles and methods available, tailored for different patients and patient situations. A variety of patient slings are available to suit different patient conditions and different purposes, e.g. transferring v. showering v. toileting. Different types of slings and models of lifters are suitable for different patient weights, including bariatric lifters for obese patients.
Risk to patient safety if not used correctly
The use of an incorrect lifter or sling, whether due to patient weight or transfer purpose, carries a significant risk of a patient fall and injury. The patient themselves must also be assessed for suitability to be lifted, and mechanised lifting is contraindicated in patients who have dementia or are otherwise uncooperative.
Three years of TGA adverse event reports relating to patient lifters
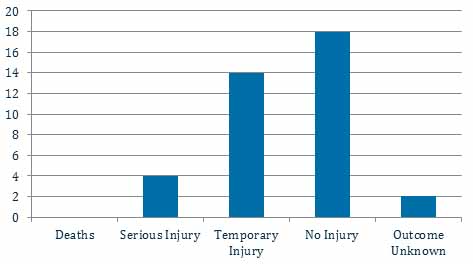
In the three years to 5 September 2018 the TGA has received four reports of serious injury and 14 reports of temporary injury to patients from using patient lifters, predominantly from falls.
Care must be taken to ensure that the lifting components are appropriately and securely attached to the lifter. Failure to do so may result in a component coming loose and a patient fall.
Staff should be reminded to always read and follow the manufacturer's instructions.
References
- Devastating injuries in healthcare workers: description of the crisis and legislative solution to the epidemic of back injury from patient lifting. Edlich RF et al, J Long Term Eff Med Implants. 2005;15(2):225-41.
 What to report? Please report adverse events, as well as near misses
What to report? Please report adverse events, as well as near misses
The TGA encourages the reporting of any suspected adverse event or potential adverse event relating to a medical device. Adverse events can involve actual harm to a patient or caregiver, or a near miss that may have resulted in harm.
Some issues relating to medical devices that may lead to adverse events and prompt you to report include:
- mechanical or material failure
- design issues
- labelling, packaging or manufacturing deficiencies
- software deficiencies
- device interactions
- user/systemic errors.
Suspected adverse events or near misses can be reported directly to the TGA:
- online at Report a problem
- by emailing iris@tga.gov.au
- by mail to IRIS, TGA, PO Box 100, Woden ACT 2606
- by fax to 02 6203 1713.
For more information about reporting, visit www.tga.gov.au or contact the TGA's Medical Devices Branch on 1800 809 361.
Disclaimer
The Medical Devices Safety Update (MDSU) is aimed at health professionals and is intended to provide practical information on medical device safety, including emerging safety issues. The information in the MDSU is necessarily general and is not intended to be a substitute for a health professional's judgment in each case, taking into account the individual circumstances of their patients. Reasonable care has been taken to ensure that the information is accurate and complete at the time of publication. The Therapeutic Goods Administration gives no warranty that the information in this document is accurate or complete, and does not accept liability for any injury, loss or damage whatsoever, due to negligence or otherwise, arising from the use of or reliance on the information provided in this document.
© Commonwealth of Australia 2018
This work is copyright. You may reproduce the whole or part of this work in unaltered form for your own personal use or, if you are part of an organisation, for internal use within your organisation, but only if you or your organisation do not use the reproduction for any commercial purpose and retain this copyright notice and all disclaimer notices as part of that reproduction. Apart from rights to use as permitted by the Copyright Act 1968 or allowed by this copyright notice, all other rights are reserved and you are not allowed to reproduce the whole or any part of this work in any way (electronic or otherwise) without first being given specific written permission from the Commonwealth to do so. Requests and inquiries concerning reproduction and rights are to be sent to the TGA Copyright Officer, Therapeutic Goods Administration, PO Box 100, Woden ACT 2606 or emailed to tga.copyright@tga.gov.au.
For the latest information from the TGA, subscribe to the TGA Safety Information email list.
For correspondence or further information about Medical Devices Safety Update, contact the TGA's Medical Devices Branch at iris@tga.gov.au or 1800 809 361.
Medical Devices Safety Update is written by staff from the Medical Devices Branch.
Editor: Ms Pamela Carter
Deputy Editor: Mr Aaron Hall
TGA Chief Medical Adviser: Adjunct Professor Tim Greenaway
Contributors include: Dr Jorge Garcia, Ms Sarah MacNaught, Ms Lisa Franklin
Supporting documents
Print version
Related content
-
Surgical staplers: TGA reviews device incident reports
Safety updatesHealth facilities and staff are reminded of the importance of following the Instructions for Use (IFU) for surgical staplers -
Updated cleaning instructions for long-term nasogastric tubes
Safety updatesChanges to the cleaning instructions for long-term nasogastric tubes have been implemented following a TGA investigation -
Consider the whole picture when testing troponin levels
Safety updatesHealth professionals should be alert to the potential for false positive results and the associated risk of unnecessary patient treatment

