Name of the ingredient
Palmidrol (AAN)
Definition of the ingredient
The substance is N-(2-Hydroxyethyl)hexadecanamide (N-palmitoylethanolamine, PEA).
Palmidrol is an endogenous fatty acid amine. Palmidrol synthesis is conducted by dehydration of the hot molten salt of ethanolamine palmitate, in solvent free condition and ethanolamine excess. The crude reaction product thus obtained is further purified by crystallization from alcohol.
Molecular formula: C18H37NO2
Structure: 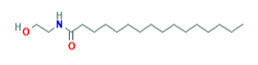
CAS Number: 544-31-0
| Test | Method reference | Acceptance criteria |
|---|---|---|
| Description | ||
| Appearance | Visual | White crystalline powder |
| Characteristics | ||
| pH | Ph. Eur 2.2.3 Potentiometric determination of pH | 6.0-.5 ( water suspension at 100mg/mL) |
| Colour | Ph Eur 2.2.25 A 1%/1cm/300nm 1% solution in isopropyl alcohol heat up to solubilisation | <0.005 AU |
| Smell | Free of ammonia and amine | |
| Solubility | Product suspended in solvents listed. | Almost insoluble in water ≥5mg/ml in DMSO ≥10mg/ml in n-octanol ≥10mg/ml in warm ethanol |
| Loss on Drying | USP <731> | <0.5% |
| Particle Size | Direct light scattering | 10 - 100µm |
| Identification | ||
| TLC | Ph Eur 2.2.27 (Thin-layer chromatography) | The principal spot in the chromatogram obtained for the sample solution is in a similar position to the Palmidrol standard solution |
| Melting Point | Ph Eur 2.2.15. (Melting point - open capillary method) | 98-102°C |
| IR Spectrum | Ph Eur (2.2.24 Absorption spectrophotometry infrared). | IR absorption maxima correspond in position and relative intensity to those in the spectrum obtained with the palmidrol standard |
| Assay | ||
| Palmidrol | HPLC Method | 98-102% w/w |
| Test | Method reference | Acceptance criteria |
|---|---|---|
| Residual solvents | ||
| Isopropanol | Ph Eur 2.4.24 | < 1000 ppm (Isopropanol) |
| Incidental metals and non-metals | ||
| Total Heavy Metals | Ph Eur 2.4.8 | Total Heavy Metals <10ppm |
| Aluminium | ICP-OES | Not more than 100 ppm |
| Nickel | ICP-OES | Not more than 30 ppm |
| Other organic or inorganic impurities or toxins | ||
| Free Palmitic acid (PA) | HPLC | ≤0.50% w/w |
| Myristoylethanolamide (MEA) | HPLC | Myristoylethanolamide (MEA) ≤0.20% w/w |
| Stearoylethanolamide (SEA) | HPLC | Stearoylethanolamide (SEA) ≤0.20% |
| Each other unknown impurity | HPLC | ≤0.10% w/w for each unknown |
| Total impurities | HPLC | ≤2.00% w/w |
| Free ethanolamine | HPLC | <0.3% (Spectrophotometric, OPA- derivative) <1.0% w/w |
| Ash (sulfuric) | Ph Eur 2.4.14 | <0.1% w/w |
Key to abbreviations
BP = British Pharmacopoeia
HPLC = High-pressure liquid chromatography
IR = Infrared spectrophotometry
PCBs = Polychlorinated biphenyls
Ph Eur = European Pharmacopoeia
TLC = Thin layer chromatography
USP = United States Pharmacopoeia

