Name of the ingredient
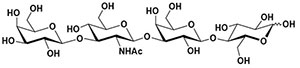
Lacto-N-tetraose (AAN #140330)
Definition of the ingredient
Lacto-N-tetraose is a purified, white to off-white amorphous powder produced by a microbial process that involves the genetically modified strain, Escherichia coli K-12 DH1.
Molecular formula: C26H45NO21
CAS Number: 14116-68-8
| Test | Method reference | Acceptance criteria |
|---|---|---|
| Description | ||
| Appearance | ISO 6658 | Powder or agglomerates, powder with agglomerates |
| Colour | ISO 6658 | White, white to off-white, off-white |
| Characteristics | ||
| pH in 5% solution (20°C) | Ph. Eur. 2.2.3 | 4.0 to 6.0 |
| Water | Karl-Fischer | ≤ 6.0 % w/w |
| Identification | ||
| NMR | Strecker et al. 1989[1] | Recorded NMR spectra must be in full agreement with the structure of LNT |
| MS |
Chai et al., 2001[2] Pfenninger et al., 2002a[3] Pfenninger et al., 2002a[4] |
Base molecular ion peak matches molecular weight(s) |
| HPLC | HPLC[5] | The retention time of the main component corresponds to the retention time of the LNT standard ±3% |
| HPAEC | HPAEC[6] | The retention time of the main component corresponds to the retention time of the LNT standard ±3% |
| Assay | ||
| Lacto-N-tetraose (water-free) |
HPLC[5] and HPAEC[6] | ≥70.0% w/w |
| D-Lactose | HPAEC[6] | ≤12.0% w/w |
| Lacto-N-triose II | HPAEC[6] | ≤10.0% w/w |
| para-Lacto-N-hexaose-2 | HPAEC[6] | ≤3.5% w/w |
| Lacto-N-tetraose fructose isomer | HPLC[7] | ≤1.0% w/w |
| Sum of other carbohydrates | HPAEC[6] | ≤5.0% w/w |
| Human-identical milk saccharides (water-free)a | Calculated theoretically as the summation of percentages of lacto-N-tetraose, lactose, and lacto-NN-triose II. | ≥90.0% w/w |
| Notes | ||
| aHuman-identical milk saccharides is defined here as the sum of lacto-N-tetraose, lactose, and lacto-N-triose II | ||
| Test | Method reference | Acceptance criteria |
|---|---|---|
| Incidental metals and non-metals | ||
| Lead | EN 13805:2002; EPA 6020A:2007 | ≤ 0.1 mg/kg |
| Other organic or inorganic impurities or toxins | ||
| Ash, sulphated | Ph Eur 2.4.14 | ≤ 0.5% w/w |
| Residual Proteins by Bradford Assay | Bradford Assay | ≤ 0.01% w/w |
| Residual Endotoxins | Ph Eur 2.6.14 | ≤ 10 E.U./mg |
| Microbiology | ||
| aWhile substance manufacturers are encouraged to include limits for objectionable microorganisms, it is the product into which those substances are formulated that is subject to a legally binding set of criteria. The Therapeutic Goods Order No. 100 'Microbiological Standards for Medicines' mandates that any finished product that contains the ingredient, alone or in combination with other ingredients, must comply with the microbial acceptance criteria set by Clause 11 of the Order | ||
Footnotes
| [1] | Strecker, G., Wieruszeski, J. M., Michalski, J. C., & Montreuil, J. (1989). Assignment of the 1H- and 13C-NMR spectra of eight oligosaccharides of the lacto-N-tetraose and neotetraose series. Glycoconjugate Journal, 6(1), 67-83. |
|---|---|
| [2] | Chai, W., Piskarev, V., & Lawson, A. M. (2001). Negative-ion electrospray mass spectrometry of neutral underivatized oligosaccharides. Analytical Chemistry, 73(3), 651-657. |
| [3] | Pfenninger, A., Karas, M., Finke, B., & Stahl, B. (2002a). Structural analysis of underivatized neutral human milk oligosaccharides in the negative ion mode by nano-electrospray MS n (Part 1: Methodology). Journal of the American Society for Mass Spectrometry, 13(11), 1331-1340. |
| [4] | Pfenninger, A., Karas, M., Finke, B., & Stahl, B. (2002b). Structural analysis of electrospray MS n (part 2: application to isomeric mixtures). Journal of the American Society for Mass Spectrometry, 13(11), 1341-1348. |
| [5] | HPLC: Column: TSKgel Amide-80 (150 x 4.6 mm); mobile phase: Water/Acetonitrile (gradient); Flow rate: 1.0 mL/min; Column temperature 25°C; UV detector 205 nm. |
| [6] | HPAEC: Column: Carbopac PA200 (3 x 250 mm with 3 x 50 mm guard column); mobile phase: 500 mM NaOH/Water/100 mM NaOH (Gradient); Flow rate: 0.5 mL/min; Column temperature 27°C. |
| [7] | HPLC: Column: Shodex HILICpak VG-50 4E (4.6 mm x 250 mm, 5 mm particle size); mobile phase: Water/Acetonitrile (gradient); Flow rate: 1.0 ml/min; Column temperature: 30°C; CAD detector. |
Key to abbreviations
EU = Endotoxin units
EN = European norms
EPA = United States Environmental Protection Agency
HPLC = High-pressure liquid chromatography
HPAEC = High performance anion exchange chromatography
ISO = International organisation for standardisation
MS = Mass spectrometry
NMR = Nuclear magnetic resonance
Ph Eur = European Pharmacopoeia
| Version | Description of change | Effective date |
|---|---|---|
| 1.0 | Initial | 17/11/2021 |
Product types

