The government is now operating in accordance with the Guidance on Caretaker Conventions, pending the outcome of the 2025 federal election.
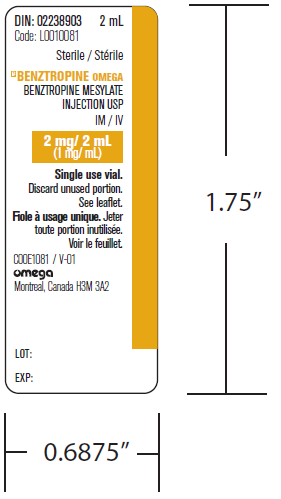
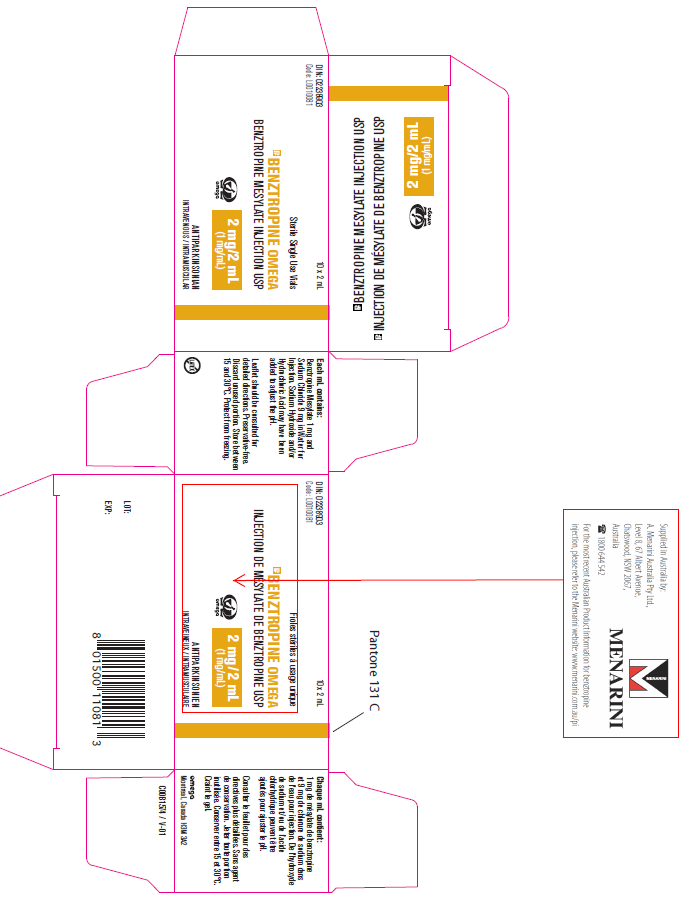
(Approval lapsed 21 March 2018) BENZTROPINE OMEGA, benztropine mesylate2mg/2ml injection vial
Benztropine OMEGA is recommended for all etiologic groups of parkinsonism - arteriosclerotic, postencephalitic, idiophatic and drug-induced. It can be effective at any stage of the disease, even when a patient has become bedridden. Often it is helpful in patients who have become unresponsive to other agents. Therapy is directed toward control of disturbing symptoms to permit maximum integration of function and minimum discomfort. In non-drug-induced parkinsonism, partial control of symptoms is the usual therapeutic accomplishment. Benztropine OMEGA is a powerful anticholinergic agent, mainly effective in relieving tremor and rigidity.