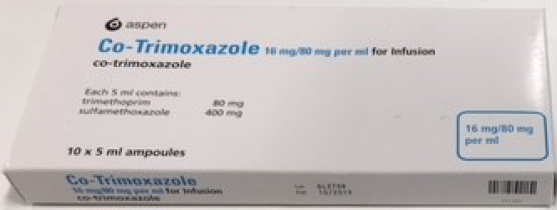
(Approval lapsed) Co-Trimoxazole 16 mg/ 80 mg per ml for Infusion (5 mL ampoules)
Section 19A approved medicine
(Approval lapsed) Co-Trimoxazole 16 mg/ 80 mg per ml for Infusion (5 mL ampoules)
Section 19A approval holder
Link Medical Products Pty Ltd ABN 73 010 971 516
Phone
1800 181 060
Approved until
Status
Expired
Medicines in short supply/unavailable
DBL SULFAMETHOXAZOLE 400 mg AND TRIMETHOPRIM 80 mg CONCENTRATE INJECTION BP 5mL injection ampoule - ARTG 16293
Indication(s)
Parenteral administration of Sulfamethoxazole 400 mg and Trimethoprim 80 mg Concentrate Injection is indicated where oral dosage is not desirable or practical, e.g. pre- and post-operative infections associated with surgery, trauma or gynaecology; septicaemia and other infections due to sensitive organisms such as typhoid and paratyphoid.
Images