Most Australians are unaware that low dose codeine (<30 mg) containing medicines used for pain relief offer very little additional benefit when compared with - medicines without codeine. The use of such medicines however, is associated with high health risks, such as developing tolerance or physical dependence on codeine. Tolerance occurs when codeine becomes less effective and so the body needs higher and higher doses to feel the same relief from your symptoms.
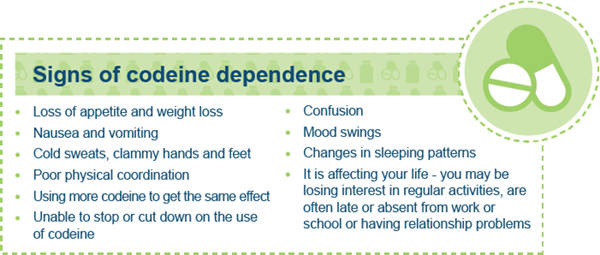
Severe withdrawal symptoms can result when the medicine is stopped; these include head and muscle aches, mood swings, insomnia, nausea and diarrhoea. Some of these withdrawal symptoms, such as head or muscle aches mimic the symptoms that low-dose codeine products are often used to treat, leading to people incorrectly continuing to take the medicine longer or in higher doses.
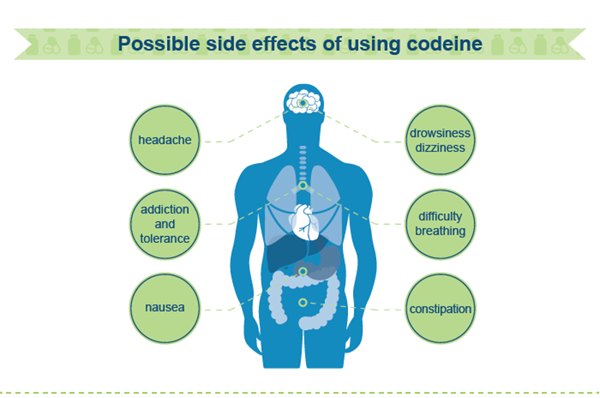
Codeine poisoning contributes to both accidental and intentional deaths in Australia. Low-dose codeine-containing medicines are usually combined with either paracetamol or ibuprofen. Long term use of high doses of paracetamol can also result in liver damage and the most severe adverse effects of long term ibuprofen use include serious internal bleeding, kidney failure and heart attack.
Codeine is also sometimes used in medicines to relieve the symptoms of cough and cold, however there are safer and more effective medicines available that may provide relief from these conditions. Talk to your pharmacist or doctor for advice on what may be best for you.
Further information on the health risks of low-dose codeine medicines can be found on the TGA website:
- George report: Review of the efficacy and safety of over-the-counter codeine containing analgesics for pain and codeine based antitussives
- Safety review: Codeine use in children and ultra-rapid metabolisers
- Codeine RIS
- Codeine harm and efficacy literature
Additional information on codeine harm can be found on:
|  |
 | |
| |
International regulation of codeine
Australia is not unique in its requirements to have a prescription for codeine containing products. Countries in Europe including Austria, Belgium, France, Germany and Italy, well as the United States, Japan, Russia, and the United Arab Emirates all require prescriptions for medicines containing codeine. In some other countries including Hong Kong, Hungary and the Netherlands, OTC sale of cough linctus (containing codeine) is allowed, with all other medicines containing codeine requiring a prescription. Most recently the new French Government announced in July 2017 that all medicines containing codeine would only be available via prescription.
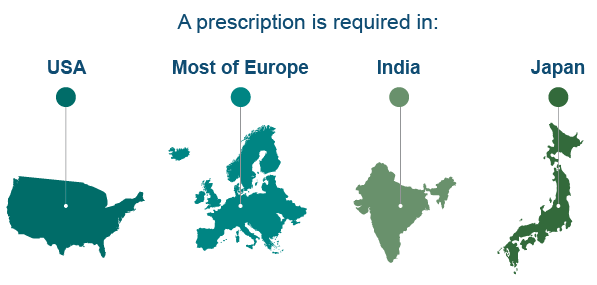
Most recently, in January 2018, the Kenya Pharmacy and Poisons Board announced that all medicines containing codeine have been rescheduled to Prescription Only Medicines. Canada and New Zealand are also currently considering whether to make codeine medicines Prescription Only.

