Related resources
This user guide follows the related information on installing the Adverse Drug Reaction (ADR) report template into Medical Director.
Open the template
- Open Medical Director.
- Open the relevant patient record.
- Open the Letter Writer (
 or F8).
or F8). - Select 'File'; 'New', as shown in figure 1.

- Select 'MD ADR Reporting Form' from the list, as shown in figure 2.
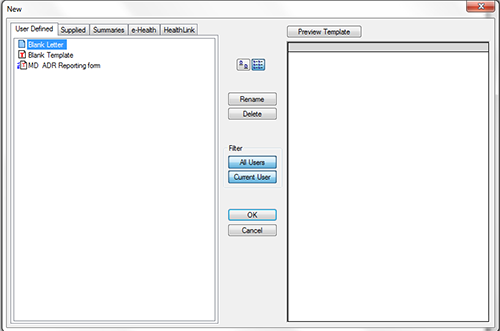
- Select 'OK'
Fill out the template
A number of prompts will appear in boxes as follows:
Medications
Select all current medicines (suspected and not suspected) and select 'OK', as shown in figure 3.

Details of the adverse drug reaction
The 'User defined fields' provides fields to enter details about the adverse event reaction. Fields can be navigated using TAB.
This box includes the following fields, as shown in figure 4:
- Patient identifier (please use patient initials only)
- Suspect drugs - provide details (including trade name) of the suspect drugs, or provide details in the fields below for each suspected drug:
- Commencement date
- Trade name
- Route of administration
- ADR Narrative - describe the adverse event in as much detail as possible. Provide the following information, either in the narrative or in the fields provided:
- Onset of reaction
- Patient symptoms
- Severity - choose from the following options:
- Congenital anomaly/birth defect
- Death
- Caused prolonged inpatient hospitalisation
- Incapacity/disability
- Life threatening
- Other
- Treatments (if applicable)
- Outcome
- Sequelae
- Other relevant information.

Edit the report
Once you have filled out all the fields, the information will be populated into the report. Edit the letter as required, noting the following:
- Add any useful and relevant information to the report.
- Additional suspect drugs can be entered - highlight one of the rows in the table, right click and select 'copy'. Place the curser below the table, right click and select 'paste'. Edit the contents of the new row with the details of the additional suspect drug.
- Remove patient identifiers or irrelevant information - for example:
- remove identifiers that have auto-populated
- only the most recent observations are needed, therefore, previous observations may be removed.
- Ensure that an email address has been included in the signature block - this may be a personal or practice email address as appropriate.
Save this instance of the report to the patient's record.
Save to file and email to the TGA (preferred)
Convert the report to either Word document or PDF as described below, to be emailed to the TGA. Reports may also be provided by fax.
PDF - Convert the report to a pdf by selecting 'file'; 'print'. Select your PDF creation software and select 'print'.
Word - Convert the report to a Word document by selecting 'file'; 'save as file' and ensure the 'save as type' field shows the latest version of Word (do not use the default 'rich text format').
Print the file to paper and send to the TGA (alternative)
Fax - Print the report to paper using your printer.
If you are scanning your report, please select the option that provides for Optical Character Recognition (OCR) results where possible. Scanning software may provide an OCR choice or similar such as 'text', 'text and graphics', 'document', or other description option. This allows reports to be processed more easily compared with reports that come to us as an image.
Send the report to the TGA
Email or fax the completed report to the TGA:
- Email (preferred): ADR.Reports@tga.gov.au
- Fax: 02 6232 8392
- Ensure faxed reports include an email address for the TGA to provide timely receipt of the report and/or seek further information.
| Version | Description of change | Author | Effective date |
|---|---|---|---|
| V1.0 | Original publication | Technical and Safety Improvement | October 2018 |

