The government is now operating in accordance with the Guidance on Caretaker Conventions, pending the outcome of the 2025 federal election.
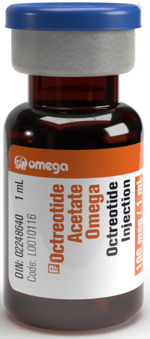
Octreotide Acetate Omega 100mcg/mL solution for injection single use vials (Canada)
Section 19A approved medicine
Octreotide Acetate Omega 100mcg/mL solution for injection single use vials (Canada)
Section 19A approval holder
Generic Health Pty Limited ABN 93 110 617 859
Phone
03 9809 7900
Approved until
Status
Current
Medicines in short supply/unavailable
OCTREOTIDE GH octreotide as acetate 100 micrograms/1 mL injection ampoule - ARTG 148402
Indication(s)
- For symptomatic control and reduction of growth hormone and IGF-1 plasma levels in patients with acromegaly, including those who are inadequately controlled by surgery, radiotherapy, or dopamine agonist treatment. Octreotide treatment is also indicated in acromegalic patients unfit or unwilling to undergo surgery, or in the interim period until radiotherapy becomes fully effective.
-
For the relief of symptoms associated with the following functional tumours of the gastro-entero-pancreatic endocrine system:
- Carcinoid tumours with features of the carcinoid syndrome;
-
Vasoactive intestinal peptide secreting tumours (VIPomas).
Octreotide is not curative in these patients.
- For reduction of the incidence of complications following pancreatic surgery.
Images