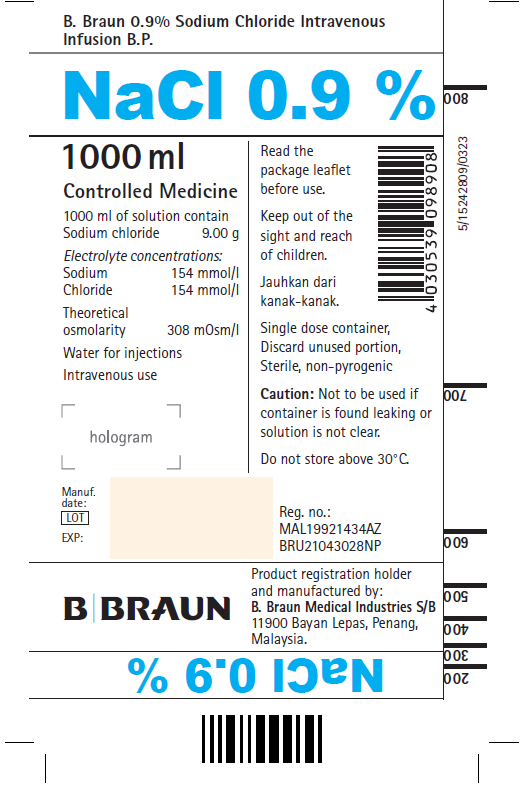
0.9% Sodium Chloride Intravenous Infusion B.P. 1000 mL Bottles (B. Braun, Malaysia)
Section 19A approved medicine
0.9% Sodium Chloride Intravenous Infusion B.P. 1000 mL Bottles (B. Braun, Malaysia)
Section 19A approval holder
B Braun Australia Pty Ltd ABN 56 002 945 155
Phone
02 9629 0200
Approved until
Status
Current
Indication(s)
Indicated for intravenous fluid therapy designed to correct deficiencies in hydration, electrolyte and energy levels. The solution may also be used as solvents for intravenously administered drugs where compatibility has been established.
0.9% sodium chloride injection may also be used for irrigation of wounds and moistening of wound dressings.
Images