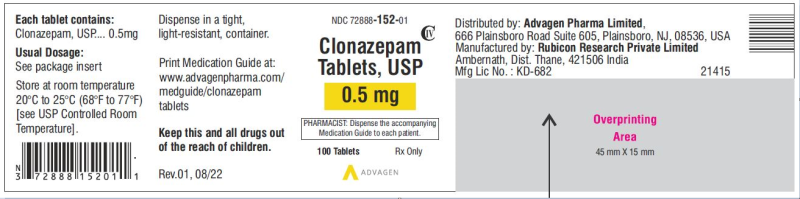
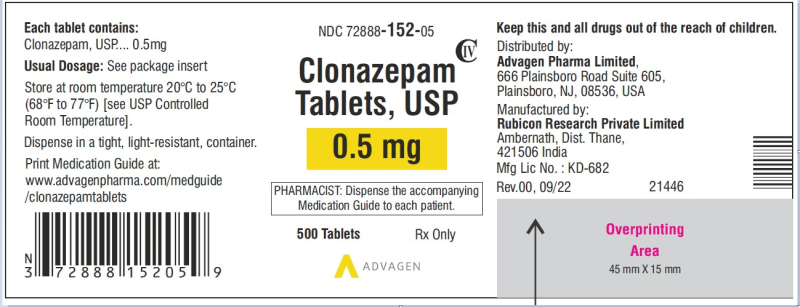
Clonazepam Tablets, USP 0.5mg (Advagen Pharma, USA)
Most types of epilepsy in children, especially absences (petit mal), myoclonic seizures and tonic-clonic fits, whether due to primary generalised epilepsy, or to secondary generalisation of partial epilepsy. In adults, all varieties of generalised epilepsy (including myoclonic, akinetic, tonic and tonic-clonic seizures), and in partial epilepsy (including psychomotor seizures).