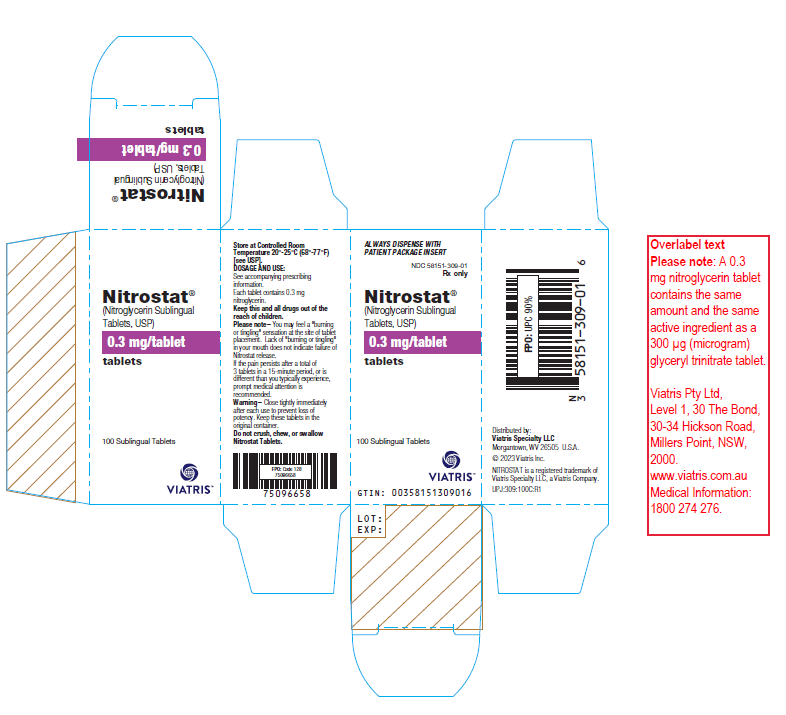
Nitrostat 0.3mg nitroglycerin sublingual tablets (USA)
Section 19A approved medicine
Nitrostat 0.3mg nitroglycerin sublingual tablets (USA)
Section 19A approval holder
Viatris Pty Ltd ABN 50 629 389 911
Phone
1800 675 229
Approved until
Status
Current
Medicines in short supply/unavailable
ANGININE glyceryl trinitrate 600microgram tablet bottle - ARTG 227783
Indication(s)
Nitroglycerin is indicated for the acute relief of an attack or acute prophylaxis of angina pectoris due to coronary artery disease.
Images