You are here
Medicines and vaccines post-market vigilance - statistics for 2014
Foreword
The Therapeutic Goods Administration (TGA) is responsible for regulating medicines, vaccines and biologicals, including monitoring the ongoing safety, quality and efficacy of these products once they have been included on the Australian Register of Therapeutic Goods (ARTG) through product vigilance activities.
On an annual basis, the TGA's Post-market Surveillance Branch prepares a report for incorporation into the Department of Health publication Australian Statistics on Medicines.
Australian Statistics on Medicines is produced by the Drug Utilisation Sub-Committee (DUSC) of the Pharmaceutical Benefits Advisory Committee (PBAC) and is aimed at providing comprehensive and valid statistics on the Australian use of medicines and vaccines in the public domain to allow access by all interested parties.
This report from the Post-market Surveillance Branch includes a brief overview on the following aspects of post-market monitoring of medicines and vaccines in Australia:
- Adverse event reporting statistics for 2014
- Processing and use of adverse event reports
- Database of Adverse Event Notifications
- 'Safety through reporting' online learning modules developed for health professionals
- Reporting adverse events
- Expert advisory committees
- Medicines Safety Update
- Product vigilance
Adverse event reporting statistics for 2014
The TGA's reporting system for adverse events began in the late 1960s with the computerised database dating back to the early 1970s. By the end of 2014 there were approximately 295,000 reports of suspected adverse events in the database.
Figure 1: Origin of medicine and vaccine adverse events received by the TGA (2010-14)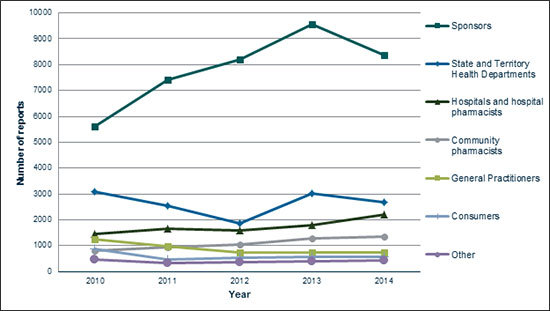
In 2014 the TGA received approximately 16,500 reports of adverse events. As shown in Figure 1, the majority of reports made in 2014 were by sponsors. The number of reports made by sponsors has significantly increased over the past five years, from 5612 in 2010 to 8359 in 2014. However, there was a slight decrease in 2014, from the peak of 9563 sponsor reports received in 2013. This change is believed to be due to factors including a focus on provision of better quality reports, following on from a strong rise the previous year after the release of new pharmacovigilance guidelines. Approximately 51% (8359) of adverse event reports received by the TGA in 2014 were from sponsors, 16% (2672) from State and Territory Health Departments (reports of adverse events following immunisation), 13% (2184) from hospitals and hospital pharmacists, 8% (1351) from community pharmacists, 5% (735) from general practitioners (GPs), 3% (565) from consumers and 3% (441) from other sources.
The numbers of reports made by sources other than sponsors in 2014 were generally similar to those received in 2013, with reports from State and Territory Health Departments decreasing slightly (from 3011 in 2013 to 2672 in 2014) and those from hospitals and hospital pharmacists increasing (from 1794 in 2013 to 2184 in 2014).
While health professionals are encouraged to report suspected adverse events directly to the TGA, they can also report to the sponsor or manufacturer.
Processing and use of adverse event reports
The Post-market Surveillance Branch assessed adverse event reports submitted to the TGA by checking for the presence of 'minimum' details, including an individual patient, an adverse event, at least one (suspected) medicine or vaccine, and an identifiable reporter. The specific adverse event terms are identified along with the suspected, interacting or 'other' therapeutic products and these are entered into the database.
The Post-market Surveillance Branch assesses causality of adverse event(s) and in some cases requests further clinical or laboratory information from the reporter. Medical officers review serious reports and Post-market Surveillance Branch staff regularly analyse reporting data to identify potential safety signals.
Reports are forwarded to the Uppsala Monitoring Centre in Sweden, which administers the World Health Organization Collaborating Centre for International Drug Monitoring. This global database began in 1968 as a pilot program involving 10 nations, including Australia, and now receives reports from more than 80 nations.
Database of Adverse Event Notifications
Information in the publicly searchable Database of Adverse Event Notifications (DAEN) comes from reports made to the TGA by a wide range of sources, including members of the public, general practitioners, nurses, other health professionals and the therapeutic goods industry. Reports in this database start from 1 January 1971 up to three months prior to the date of access. The TGA uses this three-month period to investigate each adverse event report.
The DAEN, which was launched in 2012, was created to support better health outcomes by providing access to the information that the TGA gathers while monitoring medicine and vaccine safety in Australia.
'Safety through reporting' online learning modules developed for health professionals
The TGA and NPS MedicineWise worked together to create two interactive online learning modules designed to improve adverse event reporting by health professionals and these tools were launched in December 2014.
The 'Safety through reporting' modules were developed to increase health professionals' existing knowledge around reporting adverse events associated with therapeutic products. Some of the key features include:
- the importance of reporting adverse events
- sharing the responsibility of reporting
- how to build reporting into practice
- what happens to reports once they are submitted to the TGA.
Health professionals who complete the modules are eligible for continuing professional development points from the relevant accrediting health professional bodies.
Reporting adverse events
The TGA encourages the reporting of all suspected adverse events to medicines and vaccines available in Australia, including prescription medicines, over the counter and complementary medicines. The reporting of seemingly insignificant or common adverse events can contribute to the TGA's investigation of a potential safety signal.
The TGA particularly encourages reporting of:
- suspected adverse events involving new medicines and vaccines
- suspected medicine and vaccine interactions
- unexplained adverse events (adverse events that are not described in the Product Information)
- serious adverse events, such as those suspected of causing:
- inability to work
- admission to hospital
- prolongation of hospitalisation
- increased investigation or treatment costs
- danger to life
- birth defects
- death.
For further information about reporting suspected adverse events, visit the TGA website (click on 'Report a Problem').
Sponsors of all medicines and vaccines on the ARTG have mandatory reporting requirements regarding adverse events.
Expert advisory committees
Advisory Committee on the Safety of Medicines
The Advisory Committee on the Safety of Medicines (ACSOM) was established in January 2010 to provide expert advice to the TGA about safety issues under investigation and the appropriateness of Risk Management Plans (RMPs). RMPs outline sponsors' plans to monitor and communicate risks, and are evaluated as part of the registration process for new medicines. RMPs accompany applications for registration of high risk medicines, such as new chemical entities. RMPs characterise and pro-actively manage risks relating to a medicine over its entire life cycle. ACSOM also provides advice to the TGA on other matters related to pharmacovigilance, including the detection, assessment, understanding and prevention of adverse events. ACSOM meeting statements are published on the TGA website.
Advisory Committee on the Safety of Vaccines
Following a recommendation from the government's Review of the management of adverse events associated with Panvax and Fluvax in 2012 'to consider the current governance arrangements for monitoring and responding to vaccine safety issues in Australia and make recommendations for an improved system of governance for vaccine safety monitoring', the Advisory Committee on the Safety of Vaccines (ACSOV) was established in the Therapeutic Goods Regulations. The functions of ACSOV are to provide advice and make recommendations to the Minister for Health, the TGA and the Office of Health Protection on the safety, risk assessment and risk management of vaccines. ACSOV meeting statements are published on the TGA website.
Medicines Safety Update
The Medicines Safety Update was published six times during 2014. It was published within the Australian Prescriber magazine, as well as on the TGA website. Medicines Safety Update replaced the Australian Adverse Drug Reactions Bulletin in 2010.
The following articles were published in Medicines Safety Update during 2014:
- Quetiapine and QT prolongation
- bioCSL Fluvax - not for children under 5 years
- How you can play a vital role in medicine regulation
- Olmesartan and sprue-like enteropathy
- Codeine use in children after tonsillectomy and/or adenoidectomy
- Methoxyflurane and occupational exposure
- Bexsero meningococcal B vaccine - enhanced monitoring
- Strontium ranelate and cardiovascular and venous thromboembolic risks
- Complex regional pain syndrome and vaccines
- Azathioprine and cytomegalovirus reactivation
- Measles, mumps, rubella, varicella vaccine
- Fentanyl patches and accidental exposure in children
- Zolpidem and next day impairment
- Diclofenac and arteriothrombotic events
- Bupropion and serious cardiovascular adverse events
- Methylphenidate and priapism
- Propranolol - prescribing to patients who may be at risk of self-harm
- Valproate - fetal exposure and cognitive impairment
- Medicine shortages information resource
- Epoetin alfa (Eprex) and increased risk of pure red cell aplasia with subcutaneous administration
- Pregabalin and suicidality
- Online reporting form for consumers
- Topiramate and visual field defects
- Combined oral contraceptives and hormone replacement therapy - inflammatory bowel disease
- Metoclopramide and neurological adverse events
- Publication changes for Medicines Safety Update.
Product vigilance
The TGA applies a risk management approach to ensure therapeutic goods supplied in Australia meet acceptable standards of quality, safety and efficacy. Once a therapeutic product is approved, the TGA continues to monitor the product in the market through therapeutic product vigilance activities.
The aim of therapeutic product vigilance is to continually monitor and evaluate the safety and efficacy (performance) profile of therapeutic goods and to manage any risks associated with individual products over their life cycle. The TGA's therapeutic product vigilance framework is available on the TGA website at Therapeutic product vigilance.
The maintenance and improvement of health and safety is a shared responsibility. In addition to government and industry, health professionals, consumers and their respective associations play an important role in reporting safety related issues.
Sponsors have the primary responsibility for the safety of any therapeutic products they import into, supply in or export from Australia. Sponsors must comply with legislative requirements for therapeutic product vigilance under the Therapeutic Goods Act 1989 (the Act) and there are applicable offences and penalties under the Act for not complying. The legislative requirements for therapeutic product vigilance vary depending on the type of therapeutic good.
The TGA maintains up-to-date safety information on therapeutic products that is communicated through a variety of means to consumers and health professionals. The TGA is committed to advancing public health through market authorisation of beneficial, innovative therapeutic goods and by providing timely, evidence-based and authoritative information to allow consumers and health professionals to make informed decisions.
The TGA defines therapeutic product vigilance tools as tools designed to facilitate the collection and evaluation of information pertaining to the benefits and risks associated with the use of therapeutic products. The main product vigilance tools used by the Post-market Surveillance Branch are adverse event reports, RMPs and Periodic Safety Update Reports (PSURs).
Adverse event reports are reports of any unwanted and sometimes harmful occurrences from using medicines, vaccines or medical devices (collectively known as therapeutic goods). Importantly, adverse events related to the use of a therapeutic good are not always caused by the therapeutic good itself.
RMPs provide a summary of the known important safety information about the therapeutic product, plans to identify and characterise known or potential safety concerns and plans to minimise any identified or potential safety risk. A full outline of the scope of RMPs is above (see 'Expert advisory committee'). PSURs give an annual overview of the safety of the product, including adverse events, a summary of its registration status world-wide, actions taken for safety reasons, the world-wide usage of the product and an analysis of safety requirements. Sponsors must submit PSURs to the TGA for at least three years after registration of a product.
An important aspect of product vigilance is ensuring there are mechanisms to communicate safety information to both consumers and health professionals. To achieve this, the TGA publishes Australian Public Assessment Reports (AusPARs) about recently registered prescription medicines and vaccines on the TGA website. AusPARs outline the findings of the TGA's evaluation of a product including important effectiveness and safety information.
Each adverse event report the TGA receives is entered into a database, which is continually analysed by TGA staff to identify potential emerging problems for detailed investigation.
If the TGA identifies a safety concern relating to a medicine or vaccine, we can take regulatory action. This can include:
- informing health professionals and consumers via safety alerts, Early Warning System monitoring communications and Medicines Safety Update, which provide information and recommendations about therapeutic goods
- updating the Product Information, Consumer Medicine Information and/or product labelling with new adverse effects, precautions or warnings
- requiring post-marketing studies
- imposing limits on their use
- investigating manufacturing sites
- recalling products from the market
- suspending or cancelling products.
When a product is cancelled, details are published on the TGA website.
Version history
| Version | Description of change | Author | Effective date |
|---|---|---|---|
| V1.0 | Original publication | Post-market Surveillance Branch | 12 May 2015 |



