On this page: Summary | Background | Inspections conducted | Deficiencies observed during inspections (previously referred to as "inspection findings") | Common areas of findings | Comparisons of inspection findings over time | Appendix I: Pharmacovigilance topic areas | Appendix II: Types of inspections | Appendix III: Definition of inspection gradings
Summary
From 1 January 2020 to 31 December 2020, the Therapeutic Goods Administration (TGA) conducted a total of six pharmacovigilance inspections of Australian medicine sponsors as part of its Pharmacovigilance Inspection Program (PVIP). Fewer inspections were conducted in 2020 due to a four-month pause of the program at the start of the COVID-19 pandemic.
Six routine pharmacovigilance inspections were completed in 2020. There were no 'for-cause' inspections or re-inspections.
Inspections identified:
- 2 critical deficiencies
- 27 major deficiencies
- 16 minor deficiencies.
The number and grading of deficiencies by pharmacovigilance topic area is described in Table 1 (see Appendix I for more detail on each pharmacovigilance topic area). A Corrective and Preventative Action (CAPA) plan was developed by sponsors for all identified deficiencies and all deficiencies have either been rectified or are in the process of being resolved.
| Topic area | Critical (2) | Major (27) | Minor (16) |
|---|---|---|---|
| Management of significant safety issues | - | 5 | - |
| Collection and collation of adverse drug reactions | - | 5 | 2 |
| Management of adverse drug reactions | - | 3 | 3 |
| Ongoing safety evaluation | - | 1 | 3 |
| Management of reference safety information | 2 | 3 | 1 |
| Australian Pharmacovigilance Contact Person & Qualified Person Responsible for Pharmacovigilance in Australia (QPPVA) | - | 1 | 3 |
| Reporting serious adverse drug reactions | - | 4 | - |
| Quality management system | - | 4 | 3 |
| Post-approval commitments | - | 1 | 1 |
Background
Following a successful pilot program, the TGA's PVIP commenced on 1 September 2017.
The PVIP aims to strengthen and broaden the TGA's post-market monitoring activities and protect public health by ensuring the continued safety of medicines included on the Australian Register of Therapeutic Goods (ARTG).
Pharmacovigilance inspections allow the TGA to help sponsors meet their pharmacovigilance obligations and maintain effective and robust pharmacovigilance systems. The inspections assess the sponsor's compliance with currently applicable Australian pharmacovigilance regulations and guidelines, in particular the:
- Therapeutic Goods Act 1989 (section 28(5e), 28(5)(ca), 28(2B), 28(3), 29A and 29AA)
- Therapeutic Goods Regulations 1990 (Regulation 15A)
- Pharmacovigilance responsibilities of medicine sponsors Australian recommendations and requirements (v2.2, January 2021)
- Conditions – standard and specific, applying to registered or listed therapeutic goods (section 28 of the Act).
The TGA applies a risk-based approach to scheduling pharmacovigilance inspections. This takes into account risk factors relating to the sponsor, including their products, their pharmacovigilance system and their compliance history. The TGA also reviews data provided through the PVIP Risk Assessment Survey to help plan and schedule inspections. The PVIP Risk Assessment Survey was released to sponsors again in 2020 and sponsors are reminded that non-completion of this survey results in assignment of the highest risk score.
This metrics report covers the period from 1 January 2020 to 31 December 2020. The purpose of this report is to provide a high-level overview of inspection deficiencies including a comparison of deficiencies identified in the previous reporting periods, to assist sponsors with improving their pharmacovigilance systems and preparing for pharmacovigilance inspections. All information has been de-identified.
Inspections conducted
From 1 January 2020 to 31 December 2020, the TGA conducted six routine pharmacovigilance inspections of Australian medicine sponsors (see Appendix II for types of inspections). All inspections were pharmacovigilance system related. There were no "for-cause” inspections or re-inspections and all inspections were announced. Sponsors were selected in accordance with the TGA's risk-based approach to scheduling inspections.
Four inspections were conducted over three days and two inspections were conducted over two days. All six inspections were conducted remotely via video or teleconference, with no TGA inspectors attending company premises in person.
A variety of medicine sponsors were inspected during this period, including large and small innovator companies, as well as sponsors of generic medicines, over-the-counter and complementary medicines. Deficiencies were identified in all six inspections and all inspected sponsors received at least four major deficiencies.
Deficiencies identified during inspections were graded as critical, major or minor (see Appendix III for definitions of inspection gradings). From the six pharmacovigilance inspections conducted during the reporting period, the TGA identified:
- 2 critical deficiencies
- 27 major deficiencies
- 16 minor deficiencies

A reported deficiency may have comprised of multiple separate findings, grouped according to a high-level legislative requirement or according to a cumulative pharmacovigilance impact.
The deficiencies are discussed in the next section (see Deficiencies observed during inspections).
Deficiencies observed during inspections (previously referred to as "inspection findings”)
In this section: Critical findings | Major findings | Minor findings
Critical deficiencies
Two critical deficiencies were identified from the six inspections conducted during the reporting period with a critical deficiency identified in two separate inspections.
The critical deficiencies were both categorised under the pharmacovigilance topic area: management of reference safety information (see Appendix I). Each deficiency comprised multiple findings that contributed to a failure, or significant delay, to provide patients with up-to-date information on known product risks via the product labels and reference safety information.
A critical grading was applied to each of these deficiencies because it represented a serious violation of applicable pharmacovigilance legislation and guidelines, and was considered to adversely affect the rights, safety or well-being of patients and posed a potential risk to public health. Following the inspections, both sponsors developed CAPA plans, addressing the critical deficiency and proposed actions to mitigate the risk of reoccurrence. CAPA commitments are closely monitored by the TGA until they are fully implemented. Implementation of CAPA commitments for critical findings is currently in progress for both sponsors.
Sponsors are reminded that they need to have a documented process for the maintenance of reference safety information and product labels, to ensure that the available information is in line with current scientific knowledge. Sponsors must proactively implement all mandatory warning statements and must ensure that all conditions of registration are met. Sponsors must retain records pertaining to the safety of medicines, in accordance with the record-keeping requirements outlined under paragraph 28(5)(ca) of the Therapeutic Goods Act 1989.
Major deficiencies
From the six inspections conducted during this reporting period, 27 major deficiencies were identified, with at least four major deficiencies identified in every inspection. The number of major deficiencies identified per inspection ranged between four and six (see Figure 2) and the median number of major deficiencies per inspection was four.

Figure 2 in table format
| Number of major deficiencies | Number of sponsors |
|---|---|
| 4 | 4 |
| 5 | 1 |
| 6 | 1 |
Major deficiencies by topic area
The 27 major deficiencies identified during the reporting period have been grouped by overarching topics across the pharmacovigilance system. Each topic area is also made up of various sub-topics (see Appendix I).
The proportion of major deficiencies by pharmacovigilance topic area is described in Figure 3.

Figure 3 in table format
| Topic area | Proportion of major deficiencies |
|---|---|
| Management of significant safety issues | 18.2% |
| Collection and collation of adverse drug reactions | 18.2% |
| Reporting serious adverse drug reactions | 14.81% |
| Quality management system | 14.81% |
| Management of reference safety information | 11.11% |
| Management of adverse drug reactions | 11.11% |
| Post-approval commitments | 3.7% |
| Australian Pharmacovigilance Contract Person & QPPVA | 3.7% |
| Ongoing safety evaluation | 3.7% |
Summary of major deficiencies
The topics with the highest proportion of major deficiencies in the reporting period were the management of significant safety issues and the collection and collation of adverse drug reactions, with 5/27 deficiencies each (19%), followed by reporting serious adverse drug reactions and the quality management system with 4/27 deficiencies each (15%). These deficiencies are discussed in more detail in the next section (see Common areas of deficiencies).
Minor deficiencies
Sixteen minor deficiencies were identified from the six inspections conducted during the reporting period. The number of minor deficiencies identified in each inspection ranged between zero and four (see Figure 4) and the median number of minor deficiencies per inspection was three.

Figure 4 in table format
| Number of minor deficiencies | Number of sponsors |
|---|---|
| 0 | 1 |
| 2 | 1 |
| 3 | 2 |
| 4 | 2 |
Minor deficiencies by topic area
The 16 minor deficiencies identified during the reporting period have been grouped by overarching topics across the pharmacovigilance system. Each topic area is also made up of various sub-topics (see Appendix I).
The proportion of minor deficiencies by pharmacovigilance topic area is described in Figure 5.

Figure 5 in table format
| Topic area | Proportion of minor deficiencies |
|---|---|
| Quality management system | 18.75% |
| Management of adverse drug reactions | 18.75% |
| Australian Pharmacovigilance Contract Person & QPPVA | 18.75% |
| Ongoing safety evaluation | 18.75% |
| Collection and collation of adverse drug reactions | 12.5% |
| Post-approval commitments | 6.25% |
| Management of reference safety information | 6.25% |
Summary of minor deficiencies
There were four topics with the highest proportion of minor deficiencies, 3/16 deficiencies (19%), in the reporting period as follows: quality management system, management of adverse drug reactions, Australian Pharmacovigilance Contact Person & QPPVA and ongoing safety evaluation. These deficiencies are discussed in more detail in the next section (see Common areas of deficiencies).
Common areas of deficiencies
Management of reference safety information
A deficiency of any grading, related to the management of reference safety information, was observed in every inspection conducted during the reporting period, including two critical deficiencies, three major deficiencies and one minor deficiency. These deficiencies comprised multiple findings related to delays in updating the Australian Product Information (PI), Consumer Medicine Information (CMI), product packaging leaflets, product labelling and minimum PI, with new or revised safety information.
Access to current reference safety information
The TGA recommends that PI and CMI are always accessed via TGA website. Should the sponsor decide to make the PI and CMI available on other platforms, e.g. company-owned website, then they must ensure that the published versions are current, in line with the version published on TGA website.
Timelines applicable for the maintenance of the reference safety information
Safety-related variations to the Australian PI should be submitted within six months of company decision date.
Updated PI must be lodged on the TGA website within two weeks of TGA approval of the safety related variation.
Following TGA approval of the safety related variation, CMI impact assessment should be conducted to assess if CMI revision is warranted.
If CMI revision is warranted, the updated CMI document must be lodged on the TGA website, within two weeks of the date of the approved PI document. If updates to the CMI are not warranted, then the decision and the date of CMI review should be documented.
Standard procedures following safety related variations
In the post-approval stage, the sponsor should conduct a comprehensive assessment of the impact the PI revision had on the company-sponsored material, such as educational or promotional materials, or internal reference documents and manuals, e.g. Frequently Asked Questions, used by Medical Information staff to respond to medical enquiries.
In parallel, the sponsor should communicate relevant information of the revised reference safety information to all stakeholders, both internal such as pharmacovigilance, medical, regulatory, sales, and external, such as business partners, PV agreement partners, vendors and service providers. This communication will ensure that all staff are referring to the most recent version of the PI and CMI.
Updating reference safety information for the generic medicines
Sponsors of generic medicines must comply with condition of registration to align PI and CMI within one month of a safety-related update to the innovator's PI.
Collection and collation of adverse drug reactions
A deficiency in collection and collation of adverse drug reactions was observed in every inspection conducted during the reporting period. A total of five major deficiencies and two minor deficiencies were identified for this topic which represented 19% of major deficiencies and 13% of minor deficiencies.
Deficiencies were identified in the following processes:
- Collection of safety information from medical information enquiries, product quality complaints, company-sponsored websites and social media, local and international medical literature, internal company departments – including any platforms used to record interactions with customers, business partners, TGA Database of Adverse Event Notifications (DAEN) and post-registration programs (e.g. patient support programs, product familiarisation programs, market research programs etc.)
- Inclusion of relevant safety reporting provisions in all third party contractual agreements prior to the service commencing
- Reconciliation of safety information with all possible sources
- Identification and collection of special situation reports.
Sponsors should maintain a pharmacovigilance system that allows them to identify, collect and collate all information related to safety of their medicine from all possible sources. Sponsors should also exercise due diligence and develop procedures to collate accurate and complete reports of adverse drug reactions. It is important that sponsors maintain up-to-date contact information in all publically available sources and that processes are in place to monitor and act on information collected.
Management of significant safety issues
Deficiency in the management of significant safety issues was observed in five out of six inspections conducted during the reporting period, and represented 19% of major deficiencies. This deficiency comprised multiple findings related to a failure to identify significant safety issues or a failure to report significant safety issues to the TGA within 72 hours of first awareness.
Sponsors are reminded that a significant safety issue is a new safety issue or validated signal considered by you in relation to your medicine that requires urgent attention of the TGA. This may be because of the seriousness and potential major impact on the benefit-risk balance of the medicine and/or on patient or public health, which could warrant prompt regulatory action and/or communication to patients and healthcare professionals. The TGA expects sponsors to use clinical judgement when determining whether a safety issue is significant. Sponsors are reminded that evidence of the assessments should be retained including justification of a decision not to notify TGA of a safety issue.
Sponsors should have robust procedures in place to ensure that internally validated signals and suspected or confirmed quality medicine defects are promptly assessed against the Australian pharmacovigilance guidelines to ensure compliance with regulatory reporting timeframes i.e. safety information that meets the definition of a significant safety issue must be reported to the TGA within 72 hours of first awareness.
The TGA also considers significant safety issues to include safety-related actions taken by comparable international regulatory agencies, such as the addition or modification of a contraindication, warning or precaution statement to the Product Information. Therefore, sponsors should also have procedures in place to regularly screen worldwide medical literature and information published by comparable foreign regulatory agencies, for safety-related information related to their medicine. Publications containing information that meet the definition of a significant safety issue must be reported to the TGA within 72 hours.
In scenarios where potential significant safety issues are initially received by a parent/global organisation, delays in the receipt by the sponsor company of information that meet the definition of a significant safety issue are considered unacceptable and a risk to public health.
Sponsors are reminded that third party contractual agreements should include relevant safety reporting provisions that outline responsibilities related to the identification, management and reporting of significant safety issues. There should be a process to ensure the maintenance of these provisions including for example, sponsor contact details.
Quality management system
Deficiency in the quality management system of any grading was observed in every inspection conducted during the reporting period, and represented 15% of major deficiencies and 19% of minor deficiencies, one of the four topics with the highest proportion of minor deficiencies.
Sponsors should establish a quality management system that supports their pharmacovigilance system to meet their Australian pharmacovigilance requirements. These may include, but are not limited to, standard operating procedures for:
- Collecting, processing and reporting adverse drug reactions;
- Management of significant safety issues;
- Management of reference safety information;
- Pharmacovigilance training;
- Management and retention of pharmacovigilance records;
- Audit and deviation management.
Sponsors are reminded that they should provide appropriate training to all staff engaged in pharmacovigilance activities or who might receive or process safety reports.
Pharmacovigilance training should be conducted at induction of employment, with an annual refresher at a minimum for all relevant staff as appropriate based on their roles and responsibilities.
Pharmacovigilance training documents should also be retained in accordance with the Pharmacovigilance Guidelines and s.28(5)(ca) of the Therapeutic Goods Act 1989.
Management of adverse drug reactions
Deficiency in the management of adverse drug reactions were observed in every inspection conducted during the reporting period, and represented 11% of major deficiencies and 19% of minor deficiencies, one of the four topics with the highest proportion of minor deficiencies.
Deficiencies in this area have potential impact on ICSR (individual case safety report) reporting to TGA and furthermore, company signal detection activities or analysis of safety information. These deficiencies were identified in the following areas:
- Data entry and case assessments e.g. reporter causality, documentation of Aboriginal or Torres Strait Islander (ATSI) ethnicity, seriousness assessment.
- Appropriate follow up of valid and invalid ICSRs, including consent to follow up for consumer reports
- Medical Dictionary for Regulatory Activities (MedDRA) coding
- Coding of all events within a given ICSR
Reporting serious adverse drug reactions
Deficiency in reporting serious adverse drug reactions was observed in 4 out of 6 inspections conducted during the reporting period, and represented the second highest proportion (15%) of major deficiencies, along with the quality management system.
Sponsors are reminded that all serious adverse drug reactions must be reported to the TGA within 15 calendar days of first receipt by any company personnel, including any third-party personnel conducting activities on behalf of the sponsor. Sponsors should confirm that all company personnel are trained in their pharmacovigilance reporting obligations and that robust procedures are in place to ensure that all safety information is reported promptly, to allow for compliance with the 15-day reporting timeframe. Sponsors should periodically audit their pharmacovigilance agreements to ensure that business partners or contracted personnel are complying with their pharmacovigilance reporting obligations.
Ongoing safety evaluation
A deficiency of any grading in ongoing safety evaluation was observed in four out of six inspections conducted during the reporting period, including one major deficiency.
Sponsors are reminded that safety information originating from Australia must be available to the sponsor via a single access point, within Australia, for the purpose of ongoing safety evaluation. Safety monitoring activities should include a review of cumulative cases, in order to allow for a comprehensive review of potential safety issues. Furthermore, sponsors should have a process in place to describe the methodology of detection and investigation of such issues in a timely manner.
Australian pharmacovigilance contact person and Qualified Person for Pharmacovigilance in Australia (QPPVA)
A deficiency of any grading related to the role of the Australian pharmacovigilance contact person and/or the QPPVA was observed in 4 out of 6 inspections conducted during the reporting period, including 1 major deficiency.
Deficiencies in this topic area were related to:
- a failure to notify the TGA with the name and contact details of the Australian pharmacovigilance contact person within 15 calendar days of the first medicine's entry in the ARTG, or any subsequent updates to this position (e.g. new name or updated contact details)
- inadequate oversight of the pharmacovigilance system by the QPPVA.
Sponsors are reminded that they must nominate a pharmacovigilance contact person in Australia who will be responsible for fulfilling their pharmacovigilance reporting requirements. The nominated pharmacovigilance contact person must reside in Australia and should have a sound understanding of Australian pharmacovigilance reporting requirements. The Australian pharmacovigilance contact person can be nominated, or their details updated, through the TGA Business Services Portal.
Sponsors should also have a qualified person responsible for pharmacovigilance undertakings in Australia (i.e. the QPPVA). This person may be different to the Australian pharmacovigilance contact person, although ideally they are the same person. The QPPVA should ensure that the sponsor has an effective pharmacovigilance system in place to comply with Australian pharmacovigilance requirements, and have an adequate understanding of Australian and global pharmacovigilance processes, in order to have effective oversight of the entire pharmacovigilance system.
Post-approval commitments
A deficiency in compliance with post-approval commitments was observed in two out of six inspections conducted during the reporting period, with one major and one minor deficiency identified.
Sponsors are reminded of the requirement to comply with all conditions of registration, including but not limited to, the preparation and timely submission of Periodic Safety Update Reports, maintenance of Risk Management Plans and compliance with Risk Management Plan commitments. Where the Risk Management Plan outlines risk minimisation activities and / or materials, sponsor should have a clear process in place to ensure the compliance and the effectiveness of these commitments.
Comparison of inspection deficiencies over time
From the commencement of the Pharmacovigilance Inspection Program, on 1 September 2017, to 31 December 2020, the TGA has conducted 26 pharmacovigilance inspections of Australian medicine sponsors. Ten inspections were conducted in each of the reporting periods 1 September 2017 to 31 December 2018, and 1 January 2019 and 31 December 2019. Six inspections were conducted between 1 January 2020 and 31 December 2020.
Fewer inspections were conducted in 2020 as a result of a four-month pause on inspections in response to the COVID-19 pandemic.
Although the number of inspections has changed considerably in 2020, the average number of inspection deficiencies remained at 7.7 deficiencies per inspection.
A comparison of the average number of deficiencies in the 2020 reporting period (6 inspections conducted), in the 2019 reporting period and 2018 reporting period (10 inspections conducted each), by deficiency type, is described below (see Figure 6).

Figure 6 in table format
| Year | Deficiency type | Average number of deficiencies |
|---|---|---|
| 2018 | Critical | 5 |
| Major | 2.9 | |
| Minor | 0 | |
| 2019 | Critical | 4.3 |
| Major | 3.3 | |
| Minor | 0.1 | |
| 2020 | Critical | 4.5 |
| Major | 2.6 | |
| Minor | 0.3 |
In the current reporting period, the average number of critical deficiencies increased from the previous reporting period and was the highest since the commencement of the Pharmacovigilance Inspection Program.
Major deficiencies constituted the largest proportion of deficiencies across all reporting periods. All inspections resulted in multiple major deficiencies. In the current reporting period, the average number of major deficiencies slightly increased from the previous reporting period.
In contrast, the average number of minor deficiencies in the current reporting period was lower in comparison to the previous reporting periods.

Figure 7 in table format
| Year | Deficiency type | Average number of deficiencies |
|---|---|---|
| 2018 | Case collection and collation of adverse drug reactions | 1.4 |
| Management of adverse drug reactions | 0.6 | |
| 2019 | Case collection and collation of adverse drug reactions | 1.1 |
| Management of adverse drug reactions | 0.5 | |
| 2020 | Case collection and collation of adverse drug reactions | 1.5 |
| Management of adverse drug reactions | 0.83 |
For case collection and collation as well as for management of adverse drug reactions, the average number of deficiencies in the current reporting period was higher than the average number observed in previous reporting periods.

Figure 8 in table format
| Year | Average number of deficiencies |
|---|---|
| 2019 | 10.9 |
| 2020 | 0.5 |
In 2018, the topic area of reporting serious adverse drug reactions was included in the topic area of the management of adverse drug reactions. In 2019, reporting of adverse drug reactions was separated from management of adverse drug reaction into an individual category. As a consequence, no comparative data from 2018 is available.
In the current reporting period, the average number of deficiencies in reporting serious adverse drug reactions was lower than the average observed in the previous reporting period.

Figure 9 in table format
| Year | Deficiency type | Average number of deficiencies |
|---|---|---|
| 2018 | Management of significant safety issues | 1 |
| Post-approval commitments | 1 | |
| 2019 | Management of significant safety issues | 1 |
| Post-approval commitments | 0.7 | |
| 2020 | Management of significant safety issues | 0.83 |
| Post-approval commitments | 0.33 |
In relation to deficiencies in management of significant safety issues and post-approval commitments, the average number of deficiencies in the current reporting period was lower than previous reporting periods, with a more significant decrease in deficiencies in post-approval commitments.
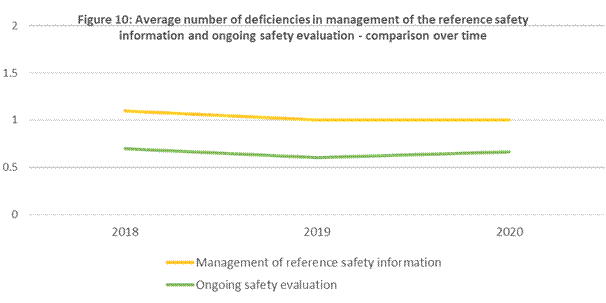
Figure 10 in table format
| Year | Deficiency type | Average number of deficiencies |
|---|---|---|
| 2018 | Management of reference safety issues | 1.1 |
| Ongoing safety evaluation | 0.7 | |
| 2019 | Management of reference safety issues | 1 |
| Ongoing safety evaluation | 0.6 | |
| 2020 | Management of reference safety issues | 1 |
| Ongoing safety evaluation | 0.66 |
The average number of deficiencies in the management of reference safety information and ongoing safety evaluation remained consistent throughout the reporting periods. It is important to note that the topic area management of reference safety information had the highest proportion of critical findings in the current reporting period (see Deficiencies observed during inspections).

Figure 11 in table format
| Year | Deficiency type | Average number of deficiencies |
|---|---|---|
| 2018 | Australian Pharmacovigilance Contact Person & QPPVA | 1.1 |
| Quality management system | 0.2 | |
| 2019 | Australian Pharmacovigilance Contact Person & QPPVA | 1.3 |
| Quality management system | 0.6 | |
| 2020 | Australian Pharmacovigilance Contact Person & QPPVA | 1.16 |
| Quality management system | 0.66 |
Whilst the average number of deficiencies in the quality management system remained consistent throughout the reporting periods, the average number of deficiencies related to the Australian Pharmacovigilance Contact Person & QPPVA was higher in the current reporting period, if compared with previous reporting periods.
Appendix I: Pharmacovigilance topic areas
| Topic area | Sub-topic |
|---|---|
| Collection and collation of adverse drug reactions | Spontaneous sources of safety data, including medical information, product quality complaints, medical literature, company personnel (e.g. sales representatives, social/digital media etc.) |
| Solicited sources of safety data, including patient support or market research programs, observational studies etc. | |
| Safety data exchange agreements | |
| Management of adverse drug reactions | Case processing, including data entry, coding, causality and seriousness assessment, and follow-up |
| Reporting serious adverse drug reactions | Reporting serious adverse drug reactions within 15 calendar days |
| Ongoing safety evaluation | Signal detection and management |
| Production of Periodic Safety Update Reports | |
| Management of significant safety issues | Identifying significant safety issues |
| Reporting significant safety issues within 72 hours | |
| Management of reference safety information | Maintenance of core safety information |
| Maintenance of Australian Product Information, Consumer Medicine Information, product packaging leaflets and product labelling | |
| Maintenance of safety-related information in company-sponsored material (e.g. educational or promotional items) | |
| Communication of updated safety-related information to internal and external stakeholders | |
| Post-approval commitments | Submission of Periodic Safety Update Reports |
| Maintenance and submission of Risk Management Plans | |
| Compliance with Risk Management Plan commitments | |
| Compliance with other conditions of registration | |
| Quality management system | Management and retention of pharmacovigilance records |
| Pharmacovigilance training | |
| Management of pharmacovigilance procedures | |
| Audit and deviation management | |
| Australian Pharmacovigilance Contact Person & Qualified Person Responsible for Pharmacovigilance in Australia (QPPVA) | Notification of the Australian Pharmacovigilance Contact Person within 15 calendar days |
| QPPVA oversight of the pharmacovigilance system |
Appendix II: Types of inspections
*excerpt from page 10-11 of the Pharmacovigilance inspection program: Guidance for medicine sponsors (Version 1.0, September 2017). Please refer to the full guidance for information on all types of inspections.
Please note the TGA is referred to as 'we' or 'us', and sponsors as 'you'.
Routine inspections
Routine pharmacovigilance inspections are scheduled as part of the inspection program. There is no specific trigger for these inspections, although we take a risk-based approach to prioritising them. These inspections are usually system-related inspections, but one or more products may be selected as examples to verify the implementation of the system and provide practical evidence of its functioning and compliance.
'For cause' inspections
'For cause' inspections are undertaken in response to specific triggers where a pharmacovigilance inspection is the appropriate way to examine the issues. 'For cause' inspections generally focus on specific aspects of the sponsor's pharmacovigilance system or examine identified compliance issues and their impact on a specific product. However, we may also inspect the sponsor's entire pharmacovigilance system as a result of a trigger. Significant public health concerns or identified noncompliance are expected to be the most common triggers.
Re-inspections
We may re-inspect the sponsor's pharmacovigilance system as part of our routine inspection program. We prioritise re-inspections by assessing risk factors. If a previous inspection identified a high level of compliance this may increase the time between re-inspections. More frequent re-inspections may occur:
- where we have identified significant noncompliance
- to verify sponsors have taken action to address deficiencies observed during inspection
- to evaluate the sponsor's ongoing compliance with their obligations and evaluate changes to their pharmacovigilance system
- when a previous inspection finds a sponsor had failed to take appropriate corrective and preventative action in response to prior inspections.
Remote inspections
These are pharmacovigilance inspections of the sponsor's premises (or the premises of a firm contracted to help fulfil the sponsor's pharmacovigilance activities) that we perform remotely using communication technology such as the internet or video/tele conferencing. If the remote inspection reveals issues that require onsite inspection, or the inspection objectives could not be met remotely, we may visit the inspection site.
Appendix III: Definition of inspection gradings
*excerpt from page 21 of the Pharmacovigilance inspection program: Guidance for medicine sponsors (Version 1.0, September 2017)
Critical deficiency:
A deficiency in pharmacovigilance systems, practices or processes that adversely affects the rights, safety or well-being of patients or that poses a potential risk to public health or that represents a serious violation of applicable legislation and guidelines.
Deficiencies classified as critical may include a pattern of deviations classified as major.
A critical deficiency also occurs when a sponsor is observed to have engaged in fraud, misrepresentation or falsification of data.
Major deficiency:
A deficiency in pharmacovigilance systems, practices or processes that could potentially adversely affect the rights, safety or well-being of patients or that could potentially pose a risk to public health or that represents a violation of applicable legislation and guidelines.
Deficiencies classified as major may include a pattern of deviations classified as minor.
Minor deficiency:
A deficiency in pharmacovigilance systems, practices or processes that would not be expected to adversely affect the rights, safety or well-being of patients.
A deficiency may be minor either because it is judged as minor or because there is insufficient information to classify it as major or critical.
Note:
- Deficiencies are classified by the assessed risk level and may vary depending on the nature of medicine. In some circumstances an otherwise major deficiency may be categorised as critical.
- A deficiency reported after a previous inspection and not corrected may be given higher classification.
| Version | Description of change | Author | Effective date |
|---|---|---|---|
| V1.0 | Original publication | Risk Management Section, Pharmacovigilance and Special Access Branch | June 2021 |

