3.5 Cetirizine hydrochloride
Part A - Interim decisions on matters referred to an expert advisory committee (November 2016)
3. Advisory Committee on Medicines Scheduling (ACMS #19)
3.5 Cetirizine hydrochloride
On this page: Referred scheduling proposal | Scheduling application | Current scheduling status | Relevant scheduling history | Substance summary | Pre-meeting public submissions | Summary of ACMS advice to the delegate | Delegate's considerations | Delegate's interim decision
Referred scheduling proposal
An application was submitted to reschedule cetirizine hydrochloride from Schedule 2 to unscheduled when in preparations for the treatment of seasonal allergic rhinitis in adults and children 12 years of age and over, when in a maximum pack size of 10 days' supply labelled with a recommended daily dose not exceeding 10 mg of cetirizine hydrochloride.
Scheduling application
This is a general application. The applicant's proposed amendments to the Poisons Standard are as follows:
Schedule 4 - Proposed Amended Entry
CETIRIZINE HYDROCHLORIDE except:
- when included in Schedule 2 ; or
- in divided preparations for oral use for the treatment of seasonal allergic rhinitis in adults and children 12 years of age and over when:
- in a primary pack containing not more than
510 days' supply; and - labelled with a recommended daily dose not exceeding 10 mg of cetirizine hydrochloride.
- in a primary pack containing not more than
Schedule 2 - Proposed Amended Entry
CETIRIZINE HYDROCHLORIDE in preparations for oral use except in preparations for the treatment of seasonal allergic rhinitis in adults and children 12 years of age an over when:
- in a primary pack containing not more than
510 days' supply; and - labelled with a recommended daily dose not exceeding 10 mg of cetirizine hydrochloride.
The applicant's reasons for the request are:
- The current proposal aligns with the recent approval for 10 mg loratadine, which is the same class of medicine as cetirizine hydrochloride and is well documented to have a very similar efficacy and safety profile to cetirizine hydrochloride, including sedation potential. This was recognised by the NDPSC in the October 2005 meeting whereby they acknowledged that both loratadine and cetirizine hydrochloride have similar CNS effects which are dose related.
Current scheduling status
Cetirizine hydrochloride is currently in Schedules 4 and 2 of the Poisons Standard as follows:
Schedule 4
CETIRIZINE HYDROCHLORIDE except:
- when included in Schedule 2 ; or
- in divided preparations for oral use for the treatment of seasonal allergic rhinitis in adults and children 12 years of age and over when:
- in a primary pack containing not more than 5 days' supply; and
- labelled with a recommended daily dose not exceeding 10 mg of cetirizine hydrochloride.
Schedule 2
CETIRIZINE HYDROCHLORIDE in preparations for oral use except in preparations for the treatment of seasonal allergic rhinitis in adults and children 12 years of age an over when:
- in a primary pack containing not more than 5 days' supply; and
- labelled with a recommended daily dose not exceeding 10 mg of cetirizine hydrochloride.
Relevant scheduling history
Cetirizine
In May 1993, the DPSSC decided to include cetirizine hydrochloride in Schedule 4 and Appendix K.
In May 1997, the NDPSC decided to include cetirizine hydrochloride in Schedule 3 as the only therapeutically active substance in divided preparations for oral use containing 10 mg or less of cetirizine hydrochloride. A limit on pack size was not considered necessary. Cetirizine hydrochloride remained in Schedule 4 except when included in Schedule 3.
In February 1998, the NDPSC decided to amend the Schedule 3 entry for cetirizine hydrochloride to include all oral formulations of cetirizine hydrochloride, when it was the only active substance in the preparation (the Schedule 3 entry was no longer to be restricted to divided preparations and the maximum dosage unit size was deleted).
At the February 1999 Meeting, the NDPSC supported a recommendation from the Trans-Tasman Harmonisation Working Party (TTHWP) that, on grounds of harmonisation, cetirizine hydrochloride in preparations for oral use be rescheduled from Schedule 3 to Schedule 2. A consequence of the deletion from Schedule 3 was the deletion of the Appendix H entry.
In November 1999, the NDPSC decided to reschedule cetirizine in all preparations for oral use to Schedule 2. The Appendix H entry for cetirizine was deleted.
In October 2005, the NDPSC considered an application to amend the working of Appendix F Part 1 for cetirizine and to remove cetirizine from oral use from Appendix K. The evidence at the time indicated that cetirizine was no more sedating than loratadine and the NDPSC agreed to alter the wording of Appendix F Part 3 and remove cetirizine for oral use from Appendix K of the SUSDP.
In June 2012, the ACMS advised that cetirizine should be exempt from scheduling, when in divided forms for oral use containing 10 mg or less of cetirizine per dose, in packs containing not more than 5 days' supply for the treatment of seasonal allergic rhinitis.
Loratadine: In July 2013, the ACMS considered a proposal to reschedule loratadine from Schedule 2 to unscheduled in oral preparations containing 10 mg or less in packs containing not more than 5 daily doses for the treatment of seasonal allergic rhinitis in adults and children 6 years of age and over, with a warning label recommending a daily dose not exceeding 10 mg loratadine for adults and children with body weight over 30 kg, or recommended daily dose not exceeding 5 mg loratadine for children with body weight 30 kg and under. The ACMS recommended that the current scheduling of loratadine remained appropriate, due to the risk of inappropriate use and delay in correct diagnosis, the lack of data on adverse effects/experiences/poisoning in Australia, no substantial public health benefit in exempting from schedules and a complicated dosage regimen with risk of inappropriate dosing.
Loratadine
In March 2016, the ACMS considered a proposal to increase the pack size of unscheduled loratadine (10 mg or less) divided oral preparations from 5 dosage units to 10 dosage units when used in adults and children 12 years of age and over for the treatment of seasonal allergic rhinitis. The delegate made a final decision (23 June 2016) that the Schedule 2 and Schedule 4 entries for loratadine be amended to increase the unscheduled loratadine dosage from 5 dosage units to 10 dosage units in divided oral preparations when used in adults and children 12 years of age and over for the treatment of seasonal allergic rhinitis with an implementation date of 1 October 2016.
Current scheduling of LORATADINE, Schedules 4 and 2:
Schedule 4
LORATADINE except:
- when included in Schedule 2; or
- in divided preparations for oral use for the treatment of seasonal allergic rhinitis in adults and children 12 years of age and over when:
- in a primary pack containing 10 dosage units or less; and
- labelled with a recommended daily dose not exceeding 10 mg of loratadine.
Schedule 2
LORATADINE in preparations for oral use except in divided preparations for the treatment of seasonal allergic rhinitis in adults and children 12 years of age and over when:
- in a primary pack containing 10 dosage units or less; and
- labelled with a recommended daily dose not exceeding 10 mg of loratadine.
For reference, current scheduling of FEXOFENADINE: Schedules 4 and 2 as follows:
Schedule 4
FEXOFENADINE except:
- when included in Schedule 2; or
- in divided preparations for oral use for the treatment of seasonal allergic rhinitis in adults and children 12 years of age and over when:
- in a primary pack containing 10 dosage units or less and not more than 5 days' supply; and
- labelled with a recommended daily dose not exceeding 120 mg of fexofenadine.
Schedule 2
FEXOFENADINE in preparations for oral use except in divided preparations for the treatment of seasonal allergic rhinitis in adults and children 12 years of age and over when:
- in a primary pack containing 10 dosage units or less and not more than 5 days' supply; and
- labelled with a recommended daily dose not exceeding 120 mg of fexofenadine.
Substance summary
Cetirizine hydrochloride (Figure 3.5) is an orally active H1-receptor antagonist and is indicated for relief of symptoms of seasonal allergic rhinitis.
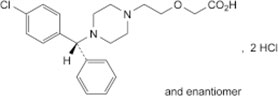
Figure 3.5: Structure of cetirizine hydrochloride
| Property | Cetirizine hydrochloride |
|---|---|
| INN/BAN/AAN | cetirizine hydrochloride |
| CAS No. | 83881-52-1 |
| Chemical name | (RS)-2-[2-[4-[(4-chlorophenyl)phenylmethyl]piperazin-1-yl]ethoxy]acetic acid dihydrochloride |
| Molecular formula | C21H27Cl3N2O3 |
| Molecular Weight | 461.8 g/mol |
| Form | white, crystalline powder |
| Solubility | water-soluble (160 g/100 mL) |
| Formulation | It is formulated as white, film-coated, scored 10 mg tablets, oral liquid 1 mg/mL and oral drops 10 mg/mL |
Pre-meeting public submissions
our (4) public submissions were received. One (1) submission supported the proposal and three (3) submissions did not support the proposal.
The main point in support was:
- The proposed increase in pack size of unscheduled cetirizine hydrochloride is supported on the basis of cetirizine hydrochloride's safety profile, for consistency with loratadine, and for consistency with other similar markets.
The main points opposed were:
- There are other options readily available and also in the best interests of consumer safety.
- Do not believe the availability of cetirizine hydrochloride in a general retail setting should be expanded. A five-day supply pack should be adequate in providing for the general goals of treatment.
- Do not believe there is substantial public health benefit in widening the scheduling exemption for cetirizine hydrochloride.
- The use of cetirizine hydrochloride in pregnancy and breastfeeding is not recommended, and loratadine or short-acting antihistamines are preferred while breast feeding.
- Consumers need to be able to discuss with a healthcare professional/pharmacist the benefits and potential risks with taking cetirizine hydrochloride in managing allergic rhinitis. With healthcare professional involvement in allergic rhinitis management, the consumer experiences better outcomes than those who set their own treatment plans.
- By allowing an exemption from scheduling, the access of cetirizine hydrochloride in an unregulated setting and where there is no healthcare professional available, this could lead to a higher incidence of adverse outcomes, negating any perceived positive benefit from greater access.
- Cetirizine hydrochloride and levocetirizine hydrochloride are currently listed on Appendix K (drugs required to be labelled with a sedation warning). If a medicine is deemed to be of sufficient risk that it must carry a warning label such as this, then it is not appropriate for sale in general retail with no access to professional advice.
- The additional sedation risk specific to cetirizine hydrochloride as evidenced by its listing on Appendix K makes it inappropriate for any further scheduling exemptions to be adopted. Based on evidence showing a higher incidence of drowsiness in cetirizine hydrochloride when compared to other second generation antihistamines such as loratadine and fexofenadine - higher risk of an adverse outcome from drowsiness - cautionary labels will not suffice.
- Lack of public need.
- Cetirizine hydrochloride is a medicine that can affect psychomotor (e.g. reaction times and hand-eye coordination) and cognitive functions (ability to make appropriate decisions), potentially having an adverse influence on the ability to drive.
- Risk increased with combined with alcohol.
- Risk of sedation and its potential impact on driving ability is best managed by facilitating access to professional advice via a Schedule 2 listing.
- Warnings on packs insufficient - 21% of Australians have driven after taking sedative OTC medicines - should be supported by verbal information from medical professionals.
- Cetirizine hydrochloride more likely to result in sedation and impairment than other non-sedating (similarly effective) antihistamines.
Summary of ACMS advice to the delegate
The committee advised that the Schedule 2 and Schedule 4 listing for cetirizine hydrochloride should be amended to include 10 days' supply.
The recommended wordings for the entries are as follows:
Schedule 4 - Amend Entry
CETIRIZINE HYDROCHLORIDE except:
- when included in Schedule 2; or
- in divided preparations for oral use for the treatment of seasonal allergic rhinitis in adults and children 12 years of age and over when:
- in a primary pack containing not more than
510 days' supply; and - labelled with a recommended daily dose not exceeding 10 mg of cetirizine hydrochloride.
- in a primary pack containing not more than
Schedule 2 - Amend Entry
CETIRIZINE HYDROCHLORIDE in preparations for oral use except in preparations for the treatment of seasonal allergic rhinitis in adults and children 12 years of age an over when:
- in a primary pack containing not more than
510 days' supply; and - labelled with a recommended daily dose not exceeding 10 mg of cetirizine hydrochloride.
The ACMS advised an implementation date of 1 June 2017.
The matters under subsection 52E (1) of the Therapeutic Goods Act 1989 considered relevant by the Committee included: a) the risks and benefits of the use of a substance; b) the purposes for which a substance is to be used and the extent of use of a substance; c) the toxicity of a substance; d) the dosage, formulation, labelling, packaging and presentation of a substance.
The reasons for the advice comprised the following:
- Adverse events associated with use of cetirizine hydrochloride during pregnancy or breastfeeding have been documented as low or nil and is managed through RASML. Increased benefit of increasing access to supply for indication, which is easy for consumers to self-diagnose, and improves patient autonomy. Rescheduling will align with other recent decisions for chemicals with a similar toxicological profile (loratadine and fexofenadine).
- The symptoms of seasonal allergic rhinitis (SAR) are easy for consumers to detect and self-diagnose and are generally short-term. It is noted that cetirizine hydrochloride may be used for other allergic disorders; however, this is unlikely to be problematic.
- Risks of cetirizine hydrochloride use include slight potential increase in somnolence compared with other less-sedating antihistamines. These effects are unlikely to be significant at the proposed doses. Adverse events associated with use during pregnancy/breast feeding have been documented but are negligible and managed by RASML requirements.
- Cetirizine hydrochloride requires a sedation warning, and dose is limited to 10 mg daily for use in adults and children 12 years of age and older.
Delegate's considerations
The delegate considered the following in regards to this proposal:
- Scheduling proposal
- ACCS advice
- Public submissions received
- Section 52E of the Therapeutic Goods Act 1989
- Scheduling Policy Framework (SPF 2015)
- other relevant information.
Delegate's interim decision
The delegate's interim decision is that the Schedule 2 and Schedule 4 entries for cetirizine hydrochloride be amended from 5 days' supply to 10 days' supply.
The proposed Schedule entry is as follows:
Schedule 4 - Amend Entry
CETIRIZINE HYDROCHLORIDE except:
- when included in Schedule 2; or
- in divided preparations for oral use for the treatment of seasonal allergic rhinitis in adults and children 12 years of age and over when:
- in a primary pack containing not more than 10 days' supply; and
- labelled with a recommended daily dose not exceeding 10 mg of cetirizine hydrochloride.
Schedule 2 - Amend Entry
CETIRIZINE HYDROCHLORIDE in preparations for oral use except in preparations for the treatment of seasonal allergic rhinitis in adults and children 12 years of age an over when:
- in a primary pack containing not more than 10 days' supply; and
- labelled with a recommended daily dose not exceeding 10 mg of cetirizine hydrochloride.
The proposed implementation date is 1 June 2017.
The matters under subsection 52E (1) of the Therapeutic Goods Act 1989 considered relevant by the delegate included: a) the risks and benefits of the use of the substance; b) the purposes for which a substance is to be used and the extent of use of a substance; c) the toxicity of the substance and d) the dosage, formulation, labelling, packaging and presentation of a substance.
The reasons for the recommendation comprised the following:
- The delegate acknowledges the committee's advice.
- The benefits outweigh the risks.
- The symptoms of seasonal allergic rhinitis (SAR) are easy for consumers to detect and self-diagnose and are generally short-term. Increased benefit of increasing access to supply for indication, and improves patient autonomy.
- Adverse events associated with use of cetirizine hydrochloride are managed through labelling.
- Rescheduling will align with other recent decisions for similar chemicals (e.g. loratadine and fexofenadine).
- Earliest possible implementation date.



