The government is now operating in accordance with the Guidance on Caretaker Conventions, pending the outcome of the 2025 federal election.
Name of the ingredient
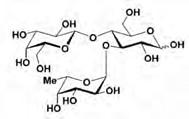
3-Fucosyllactose (AAN #140325)
Definition of the ingredient
3-Fucosyllactose (3-FL) is a white to off-white powder or agglomerates that is produced by a microbiological process (with a genetically modified strain of Escherichia coli K12 DH1). Production of 3-FL may include an optional crystallisation step.
Molecular formula: C18H32O15
CAS Number: 41312-47-4
| Test | Method reference | Acceptance criteria |
|---|---|---|
| Description | ||
|
Appearance |
ISO 6658 |
Powder, agglomerates, powder with agglomerates |
|
Colour |
ISO 6658 |
White to off-white |
| Characteristics | ||
|
pH in 5% solution (20 °C) |
Ph Eur 2.2.3 |
3.2 to 7.0 |
|
Water |
Karl-Fischer (Ph Eur 2.5.12) |
≤6.0% w/w |
| Identification | ||
|
HPLC |
HPLC[1] |
The retention time of the main component corresponds to the retention time of the standard ±3%. |
|
HPAEC |
HPAEC[2] |
The retention time of the main component corresponds to the retention time of the standard ±3%. |
|
NMR |
Ishizuka et al.,1999[3] |
Recorded NMR spectra must be in full agreement with the structure of 3-FL. |
|
MS |
MS[4] |
Base molecular ion peak matches molecular weight of 3-FL. |
| Assay | ||
|
3-Fucosyllactose (3-FL) (water- free) |
HPLC[1] |
≥87.0% w/w |
|
Assay of specified saccharides (water-free) |
Calculated theoretically as the summation of percentages of 3-FL, D- lactose, L-fucose, and 3- fucosyllactulose. |
≥92.0% w/w |
|
D-Lactose |
HPAEC[2] |
≤5.0% w/w |
|
3-Fucosyllactulose |
HPAEC[2] |
≤1.5% w/w |
|
L-Fucose |
HPLC[5, 6] |
≤1.0% w/w |
|
Sum of other carbohydrates* |
HPAEC[2] and HPLC[6] |
≤5.0% w/w |
| Notes | ||
|
*Sum of other carbohydrates is defined here as “the sum of levoglucosan, sorbitol, trehalose, lactitol, 1,6-anhydro-glucofuranose, 3-fucosyllactitol, β,β-trehalose, α,β- trehalose, galactose, glucose, 6’-β-fucosyl-lactose, isomaltose, lactulose, kojibiose, gentiobiose, isomaltotriose, cellobiose, nigerose, maltose, sophorose, laminaribiose, 6- α-glucofuranosyl-glucose, 6-β-glucofuranosyl-glucose, panose, and monoacetyl 3-FL. |
||
| Test | Method reference | Acceptance criteria |
|---|---|---|
| Residual Solvents | ||
|
Acetic acid (as free acid and/or sodium acetate)† |
Acetate kinase and phosphotransacetylase- based enzymatic assay[7] |
≤0.5% w/w |
| Incidental metals and non-metals | ||
|
Lead |
ICP-MS by EN 13805:2002; EPA-6020A:2007 |
≤0.1 mg/Kg |
|
Arsenic |
ICP-MS by EN 13805:2002; EPA-6020A:2007 |
≤0.2 mg/Kg |
|
Copper |
ICP-MS by EN 13805:2002; EPA-6020A:2007 |
≤0.5 mg/Kg |
| Other organic or inorganic impurities or toxins | ||
|
Ash, sulphated |
Ph Eur 2.4.14 |
≤0.5% w/w |
|
Residual Proteins |
Bradford Assay |
≤0.01% w/w |
|
Residual Endotoxins |
Ph Eur 2.6.14 |
≤10 E.U./mg |
| Notes | ||
|
†Relevant only for 3-FL crystallised from acetic acid. |
||
Footnotes
| [1] |
HPLC: TSKgel Amide-80 (4.6 x 150 mm); Mobile phase: Water/Acetonitrile (isocratic); Flow rate: 1.1 mL/min; Column temperature: 25°C. |
|---|---|
| [2] |
HPAEC: Column: Carbopac PA210 (4 x 150 mm with 4 x 30 mm guard column); Mobile phase: Water/500 mM NaOH/100 mM NaOH (gradient); Flow rate: 0.8 mL/min; Column temperature 31°C. |
| [3] | Ishizuka, Y., Nemoto, T., Fujirawara, M. K., F., and Nakanishi, H. (1999). Three-dimensional structure of fucosyllactoses in an aqueous solution. J. Carbohydr. Chem., 18(5), 523-533. |
| [4] | MS: Sample is dissolved in Water/Acetonitirile 1:1 (V/V); Dry temperature: 280 oC; End plate offset: 500 V, 90 nA; Capillary: 3000 V, 160 nA; Nebuliser pressure: 4 bar; Dry gas: 8.0 L/min; Ionisation: ESI negative; Mode: Full scan MS; Calibration: with Na-formate cluster solution. |
| [5] | HPLC: TSKgel Amide-80 (4.6 x 150 mm); Mobile phase: Water/Acetonitrile (isocratic); Flow rate: 1.1 mL/min; Column temperature: 25°C. |
| [6] | HPLC: Shodex Asahipak NH2P-50 4E (4.6 x 250 mm); Mobile phase: Water/Acetonitrile (isocratic); Flow rate: 1.1 mL/min; Column temperature: 25°C. |
| [7] |
As described in https://www.megazyme.com/documents/Assay_Protocol/K-ACETRM_DATA.pdf - external site |
Key to abbreviations:
EN = European Norms
EPA = United States Environmental Protection Agency
E.U. = Endotoxin Units
HPAEC = High Performance Anion-Exchange Chromatography
HPLC = High Performance Liquid Chromatography
ICP = Inductively Coupled Plasma
ISO = International Organisation for Standardisation
MS = Mass Spectrometry
NMR = Nuclear Magnetic Resonance
Ph Eur = European Pharmacopoeia



