This guidance is no longer in use and has been replaced by ARGB Appendix 13 - Guidance on TGO 109: Standards for Biologicals-General and Specific Requirements.
Introduction
Therapeutic goods order (TGO) 84 (Standards for human cardiovascular tissue) - external site applies to human cardiovascular tissue, including aortic, mitral, pulmonary and tricuspid heart valves or any part of such valves and vascular tissue (such as conduit or greater vessel graft, peripheral vascular tissue grafts and pericardial grafts). These tissues can be collected from living human donors for allogeneic use, including domino donors (that is persons who, by receiving an organ transplant, donate the removed tissue for allogeneic use) and deceased human donors for allogeneic use. TGO 84 does not apply to cardiovascular cells and tissue biopsied for the purpose of an in vitro diagnosis only and human cardiovascular tissue processed beyond minimal manipulation. Please note that heart valves are not considered 'structural tissue' for the purposes of the definition of minimal manipulation. If you are unsure if the cardiovascular order applies to a specific biological, the TGA should be contacted for clarification prior to the preparation of a dossier.
This guidance comprises notes on the interpretation of the various requirements of TGO 84, and a table (Table 1) aligning the requirements of the TGO with the dossier preparation guidance. Table 1 is designed to provide both guidance on where information may be placed in the dossier and evidence that the various requirements of the TGO have been addressed. The requirement in question should be discussed in the indicated sections of the Dossier, and that information should be summarized as evidence the requirement has been met. Please note that the TGA will evaluate the entire dossier, so only a brief summary is required. The completed table should be included with the submitted dossier as Appendix 1.
Commencement and updates
TGO 84 commences on the 31st May 2012. This will allow for a transition period for manufacturers to achieve compliance with the standards. All human cardiovascular tissue collected prior to 31st May 2012 will be exempt from this order.
TGO 84 will be subject to review on a regular basis, or as changes in technology, policy, or best practice requires. Ongoing stakeholder feedback in relation to any changes in practices or evolving technologies which may impact upon the Orders is desirable.
TGO 84 Section 7 guidance
The definition of 'critical materials' is provided in ARGB Appendix 14 - Glossary.
In order to minimise extrinsic contamination of the starting material tissue, any container or packaging material in direct contact with the tissue material should be sterile, and collection/processing staff should wear appropriate clothing.
This subsection applies to cardiovascular tissue that will not be subject to a bioburden reduction process. When this applies, the time from collection from a living donor or asystole to the commencement of cryopreservation must be no greater than 48 hours. The flowchart in Annex 1 (Figure 1) shows how the requirements identified in this subsection and subsection 7(3) establish the suitability of the collected cardiovascular tissue for therapeutic use.
Unless the entire tissue product is subject to bioburden testing, it is necessary to validate that a sample or portion is representative of the entire tissue. During sampling validation studies it is necessary to sample from a variety of areas across the tissue and to compare the bioburden test results. Routine bioburden samples should subsequently be taken from any area which represents the 'worst case' in terms of bioburden.
The microbial contamination sections of the pharmacopoeial default standards*, as well as ISO 11737-1**, and ISO 14160*** provide useful guidance on suitable bioburden test methods and their validation to demonstrate neutralisation/inactivation of antimicrobial substances. ISO 11737-1 specifically describes steps to establish the recovery efficiency and correction factor(s) to be applied when testing bioburden on or within solid and semi-solid starting materials (e.g bone, tendon, etc) and Annex A.5.3 of ISO 14160 provide guidance on performing bioburden tests on animal tissues.
*British Pharmacopoiea, European Pharmacopoeia, United States Pharmacopeia (Also see TGO 77 for medicines for section references)
**ISO 11737-1 Sterilization of medical devices - Microbiological methods - Part 1: Determination of a population of microorganisms on products.
***ISO 14160 Sterilization of health care products -Liquid chemical sterilizing agents for single-use medical devices utilizing animal tissues and their derivatives - Requirements for characterization, development, validation and routine control of a sterilization process for medical devices.
Pharmacopoieal and ISO standards for bioburden test methods include a requirement to validate the methods. This involves challenging the method used for the material or product with low numbers (< 100 cfu) of reference challenge microorganisms and recovering these organisms within the shortest test incubation time. Given that antimicrobial agents could be present in the starting materials and/or the end product, it is necessary to attempt to neutralise these agents to optimise the recovery of the challenge microorganisms, and ultimately, any product contaminants. Pharmacopoeial and ISO methods mandate this step under "suitability of test method" or "method validation". Antimicrobial activity can often be removed by filtration, dilution and/or chemical inactivation by use of a suitable neutralising agent. Tissue banks should attempt to identify antimicrobial agents used to treat donors and those agents used during processing to assist them to identify suitable neutralising agents. If, after exhaustive attempts, antimicrobial properties cannot be neutralised, then pharmacopoeias permit the product to be tested under the set of conditions established as optimal for recovery. This approach must be justified and details provided for assessment and/or audit by the TGA.
If bioburden test results fall outside the product specifications, then the tissue product should be rejected. However, if there is an urgent clinical need to use out-of-specification tissue product, then release of the product for use depends on a risk-based judgement as to whether the benefits outweigh the risks. The criteria used to inform this decision should be documented.
This subsection applies to cardiovascular tissue that will be subject to a bioburden reduction process. When this applies, the time from collection from a living donor or asystole to the treatment of antimicrobial agents must commence within 36 hours. The flowchart in Annex 1 (Figure 1) shows how the requirements identified in this subsection and subsection 7(2) establish the suitability of the collected cardiovascular tissue for therapeutic use. See subsection 7(2) for guidance on bioburden sampling, method validation (including neutralisation).
Qualification and routine control of sterilisation processes should be in accordance with the relevant parts of international (ISO) sterilisation standards. For example: ISO 11135 (ethylene oxide), ISO 14937 (general methods), ISO 17665 (moist heat), ISO 11137 (radiation), ISO 14160 (chemical sterilisation), ISO 20857 (dry heat).
Some of these standards are relevant to the sterilisation of containers and ancillary materials used during tissue processing.
The tests for heart valve competency must be detailed, and the choice of method(s) justified.
All containers should be sterile and any of the (ISO standards listed above provide guidance for validating the relevant sterilisation process for containers, or the containers could be purchased sterile. If cardiovascular tissue is harvested and processed aseptically the ISO 13408* series of standards provide useful guidance.
*ISO 13408 Aseptic processing of health care products (Note that Part 8 is being developed for the aseptic processing of cell and tissue products)
As the storage conditions for cryopreserved cardiovascular tissue are stated in the this order, that is at or below minus 100°C for no more than five years, validation of these storage conditions is not required. If storage conditions for non-cryopreserved cardiovascular tissue are implemented, full validation must be carried out.
As the transportation conditions for cryopreserved cardiovascular tissue are stated in the Cardiovascular Order, that is at or below minus 100°C, the transportation temperature does not require validation. Note that the container system for transport will require validation. If transportation of non-cryopreserved cardiovascular tissue occurs, full validation must be carried out.
Please note that in addition, all relevant requirements of TGO 87 (Labelling) also apply to Cardiovascular tissue. If further clarification is required then please contact the TGA.
Annex 1 Flow chart
Figure 1 Flow chart summarizing decision points and subsequent outcomes of TGO 84 Subsection 7(2) and 7(3)
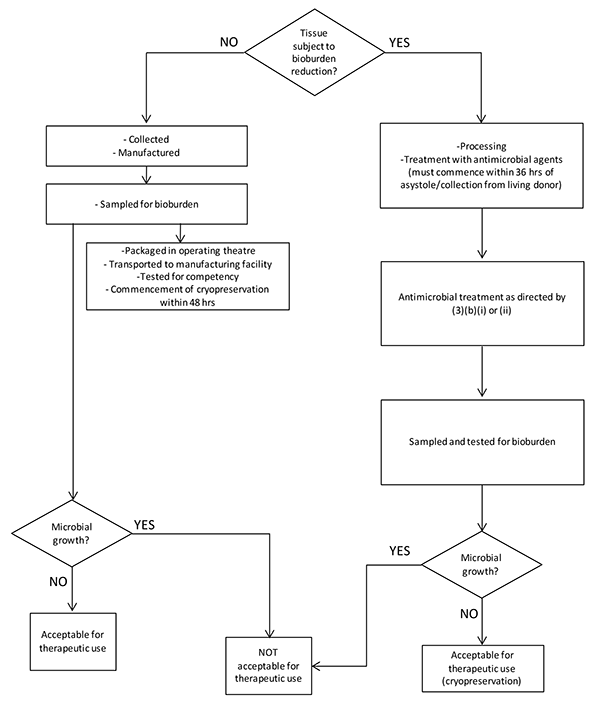
This text representation of this flowchart is provided as a list with numbered steps.
Tissue subject to bioburden reduction?
- IF NO:
- Collected
- Manufactured
- Sampled for bioburden
- Packaged in operating theatre
- Transported to manufacturing facility
- Tested for competency
- Commencement of cryopreservation within 48 hrs
- Microbial growth?
- IF NO: Acceptable for therapeutic use - end flowchart
- IF YES: NOT acceptable for therapeutic use - end flowchart
- If YES:
- Processing
- Treatment with antimicrobial agents (must commence within 36 hrs of asystole/collection from living donor)
- Antimicrobial treatment as directed by (3)(b)(i) or (ii)
- Sampled and tested for bioburden
- Microbial growth?
- IF NO: Acceptable for therapeutic use (cryopreservation) - end flowchart
- IF YES: NOT acceptable for therapeutic use - end flowchart
- End flowchart
Location of requirements in dossier
Please submit the completed table as Appendix 1 to the dossier.
| Subsection | Summary of TGO 84 requirement | Relevant dossier section/s* | Summary of how requirement is met** | Reference documents (SOPs etc) |
|---|---|---|---|---|
| 7(1) | Critical materials employed in the collection and manufacture of cardiovascular tissue | 4.1.4 (Collection) 4.2.3 (Control of critical materials) | ||
| 7(2)a | Cardiovascular tissue NOT subjected to bioburden reduction - processing time and bioburden sampling | 4.2.4 (Critical steps and intermediates) | ||
| 7(2)b | Cardiovascular tissue NOT subjected to bioburden reduction - action following bioburden sampling | 4.2.4 (Critical steps and intermediates) | ||
| 7(3)a | Cardiovascular tissue subjected to bioburden reduction - processing time | 4.2.4 (Critical steps and intermediates) | ||
| 7(3)b | Cardiovascular tissue subjected to bioburden reduction - antimicrobial treatment | 4.2.4 (Critical steps and intermediates) | ||
| 7(3)c | Cardiovascular tissue subjected to bioburden reduction - action following bioburden sampling | 4.2.4 (Critical steps and intermediates) | ||
| 7(4) | Cardiovascular tissue terminal sterilization | 4.2.5 (Validation of manufacturing process) | ||
| 7(5) | Assessment of heart valve competency prior to freezing | 4.2.4 (Critical steps and intermediates) | ||
| 7(6) | Cardiovascular tissue packaging | 4.4.6 (Containers) | ||
| 7(7) | Storage of cryopreserved cardiovascular tissue | 4.5 (Storage & Stability) | ||
| 7(8) | Transportation of cryopreserved cardiovascular tissue | 4.7 (Transportation) |
* Suggested dossier location; actual location of information may vary depending on the nature of the product, but must be defined under this heading
** Only a very brief summary is required, the entire dossier will be evaluated.
References
| Version | Description of change | Author | Effective date |
|---|---|---|---|
| V1.0 | Original | BSS | June 2011 |



