Accounts
Setup
Frequent reporters using the Adverse Event Management System (AEMS) may wish to register a personal account. As part of this registration process, reporters are required to provide the following information to successfully register an account:
- username; password; email address (for the purposes of account registration)
- personal information, such as full name, health practitioner type (if applicable), contact details.
To register an account reporters can select the 'Register' link on the top right of the portal.
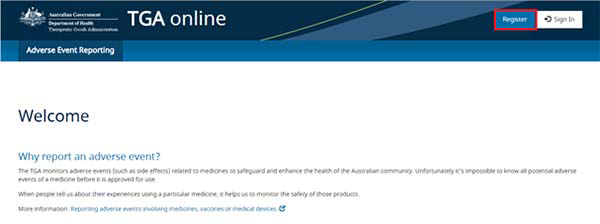
Registering an account provides health professionals and consumers who frequently report the following benefits:
- the details required in 'Step 1 - Reporter details' of the reporting form will be prepopulated from the details saved in the account profile, which will reduce the need to duplicate information if multiple reports will be submitted
- reports can be saved and edited in the 'Drafts' view until they are ready to be submitted
- a record of each submitted report will be stored in the 'Submitted' view of the Reporting Dashboard where printable summary reports can be accessed
- if the reporter needs to add follow-up information or make any changes to a submitted report, they can amend a report from the Reporting Dashboard.
Reporters will also be able to use their account credentials to login to the Special Access Scheme (SAS) portal if required. SAS accounts can also be used to login to AEMS as long as the reporter has not used a decommissioned ADRS account (see Accessing historical reports through the decommissioned Adverse Drug Reaction System (ADRS)).
Password resets
AEMS passwords will expire every 90 days. After expiry system users will need to reset their password.
If system users need to reset an expired password or they have forgotten their password and cannot sign in they reset it as follows:
From the login screen select the 'Forgotten your password?' link:
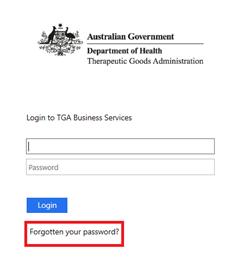
Enter their username and select 'Reset'

A password reset email will be sent to the email address associated with the account:
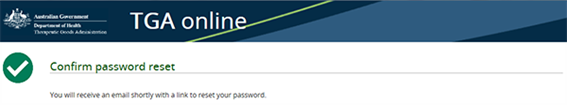
Once the password reset email has been received, click the provided link. The reporter will be required to enter a new password:

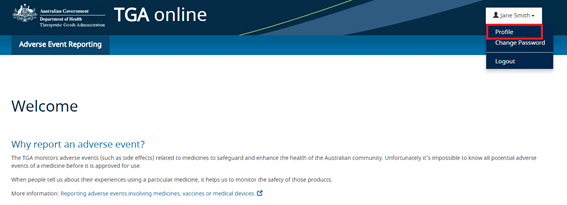
Passwords can also be reset from the AEMS portal by selecting the 'Change password' option from the dropdown menu in the reporter profile:

Updating account information
Once an account has been registered in AEMS, reporters will be able to update the information associated with their account (including name and contact details) by updating the reporter profile:
Technical difficulties
The TGA will advise registered users if the AEMS portal will be unavailable for scheduled maintenance.
In the event the AEMS portal is unavailable unexpectedly please contact adr.reports@health.gov.au.
AEMS reporting dashboard
All reporters who have registered an account in AEMS will have their own personal Reporting Dashboard that displays information specific to adverse event reports they have drafted or submitted via the system. There are two tabs which will display draft reports and submitted reports.
Draft reports
Draft reports are saved when a reporter has entered information as part of a new adverse event report but has not yet submitted this to the TGA. A draft report can be accessed from the Reporting Dashboard's 'Draft' tab to be completed and submitted at a later date.
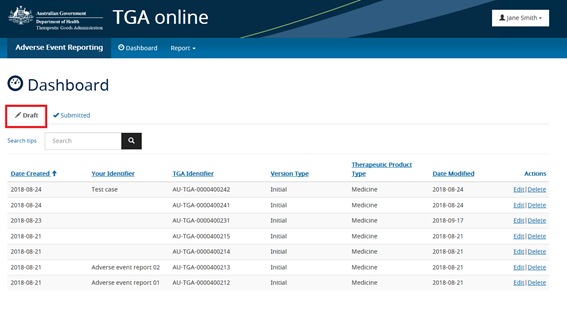
Submitted reports
All adverse event reports that have been submitted via AEMS will appear under the 'Submitted' tab. From the 'Submitted' tab reporters can generate a PDF summary of the adverse event reports, provide follow-up information by selecting 'Amend' or withdraw a report.
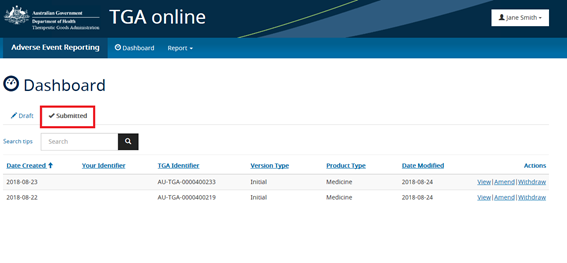
Adverse event reporting form
To report an adverse event from the AEMS portal the reporter must select 'Report > An adverse event of a medicine or vaccine' from the menu bar.
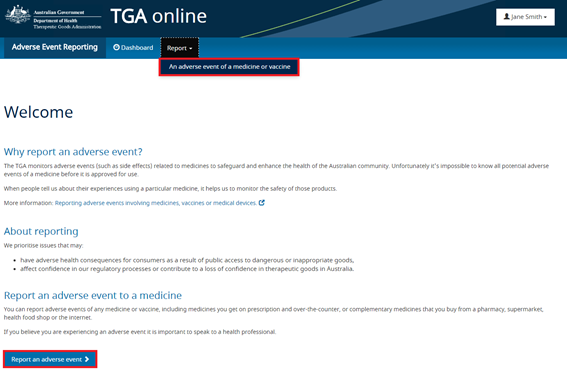
The reporting form consists of a series of six steps. All mandatory fields are marked with a red asterisk.
Step 1 - Reporter details
On this step we require contact details so that we can send the reporter a confirmation once we have received the report. We may also get in contact if we require further information.
The reporter's details will be pre-populated if the reporter is using a registered account.
The 'Your case identifier' field should be populated with the internal case identifier used by your organisation. This is not a mandatory field.
The TGA identifier for the report will be displayed in the top right-hand corner and will be displayed on each step of the form. This identifier should be used in any correspondence with the TGA.
Step 2 - Patient details
The TGA requires at least one field to be entered on Step 2. On this step the reporter should record whichever relevant details they have relating to the patient. It is the TGA's preference that the age, gender and ethnicity fields are completed.
The 'ethnicity' dropdown has been populated from the Australian Bureau of Statistics' Australian Standard Classification of Cultural and Ethnic Groups
Step 3 - Medicine details
On Step 3 the reporter can add all medicines that have been administered to the patient leading up to the adverse event. At least one medicine must be added and identified as suspected.
Once a medicine has been added, further information such as dosage and indication details can be entered by selecting the 'View, add and edit items' link under the 'Dosage and reason for use' column.
The 'Aust L' or 'Aust R' numbers can be found on a medicine's outer packaging or label. These numbers show that the medicine is accepted for supply in Australia by the TGA.
Step 4 - Reaction details
On Step 4 the reporter should add all reactions experienced by the patient. A 'reaction/symptom description' and an 'outcome' must be provided for each reaction.
Step 5 - Additional details
On Step 5 any further information can be added about the adverse event such as the sequence of events, any treatments that have been provided or any other relevant information. The reporter can also provide relevant supporting documents such as X-rays, medical reports, test reports and photographs.
Step 6 - Summary report
On Step 6 the reporter can generate a summary report of the adverse event report. A PDF document will be created that displays all recorded information. This document can be saved or printed by the reporter.
This is the final step, and once completed, the reporter can select 'Submit report'. Once submitted the reporter will be taken to a confirmation page.
Submitting follow-up information
If the initial report was submitted using the AEMS online reporting form, follow-up information can be added via the online portal. From the 'Submitted' view on your Reporting Dashboard locate the relevant report and select 'Amend'. You will then be able to add any follow-up information and submit it to the TGA.
If the initial report was submitted via any other method such as by email or the decommissioned ADRS service then follow-up reports cannot be submitted using the AEMS online reporting form. These follow-up reports will need to be submitted via email to adr.reports@health.gov.au.
Accessing the decommissioned Adverse Drug Reaction System (ADRS)
The ADRS is still accessible in a read-only format. Users can view their historical reports but will not be able to submit any new reports using this system. Users can login to the system by entering their ADRS credentials on the Login to TGA Business Services page.
These accounts cannot be used to login to the AEMS Portal. To continue reporting adverse events to the TGA you will need to access the AEMS Portal.
ADRS password resets
If you have forgotten your ADRS password or it has expired then you can reset your password by using the following steps:
From the login page selecting 'Forgotten your password?':

Then selecting the link to the ADRS password reset:

- The user can then enter their User ID and a password reset email will be sent to the email address associated with that account.
| Version | Description of change | Author | Effective date |
|---|---|---|---|
| V1.0 | Original publication | Pharmacovigilance and Special Access Branch | 07/11/2018 |
| V1.1 | Minor update to correct errors | Pharmacovigilance and Special Access Branch | 17/12/2018 |

