A list of TGA approved terminology can be found in the Ingredients Table and Code Tables in TGA Business Services (TBS). The TBS portal provides publicly visible information relating to ingredient names and approved terminology and does not require a login.
Ingredients Table
The Ingredients Table provides a list of Australian approved names for substances used in therapeutic products, including:
- active ingredients,
- excipients,
- components or 'equivalents' of ingredients,
- cells and tissues.
How to search the Ingredients Table
- Go to the Ingredients Table,
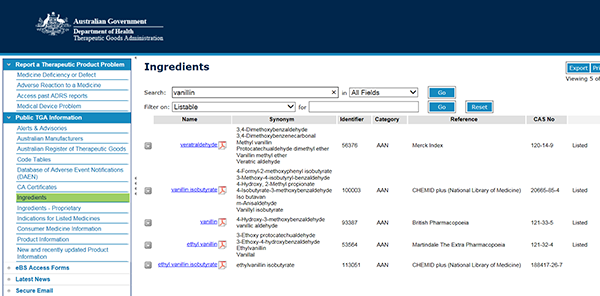
- Type ingredient name in the search box. For example, 'vanillin' and press the 'GO' button.
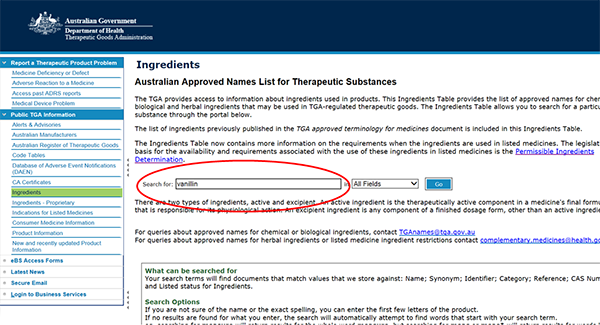
- Select the ingredient name (hyperlinked in blue, in the 'Name' column), to view the Ingredient Summary (pdf).
No search results
If no search results are returned, try searching for the CAS number. If there are still no results, you will need to follow the process for proposing a new ingredient name.
Ingredient Summary
The Ingredient Summary is a pdf document found in the Ingredients Table that provides a publicly visible summary of an ingredient and the approved role of the ingredient (active or excipient).

How to interpret an Ingredient Summary
The Ingredient Summary generally lists the following information:
Ingredient name - TGA approved ingredient name
Ingredient ID - ingredient identifier assigned by the TGA
Category - approved ingredient and tissue name categories, e.g. AAN, ABN, ACN etc.
Synonyms - other names by which the ingredient is known by (included to assist with search functionality; where applicable)
CAS number - ingredient identifier assigned by the Chemical Abstract Service (CAS); (where possible)
Naming reference - publication that served as the source of the ingredient name, e.g. INN, British Pharmacopoeia, monograph)
Restrictions - any restrictions that might apply to use or labelling of the ingredient, e.g. to be used topically, only to be used as an active homeopathic ingredient
Availability - product types and roles the ingredient is permitted for use in e.g. listed medicines, prescription medicines etc.
Availability restrictions
The ingredient availability section must be read in totality, as additional restrictions on availability can apply. For example, this screenshot shows that 'Strychnos ignatii':
- is available for use as an active ingredient in export only, prescription medicines and listed medicines
- that listed medicine availability is permitted only when the ingredient is used as a homoeopathic ingredient.

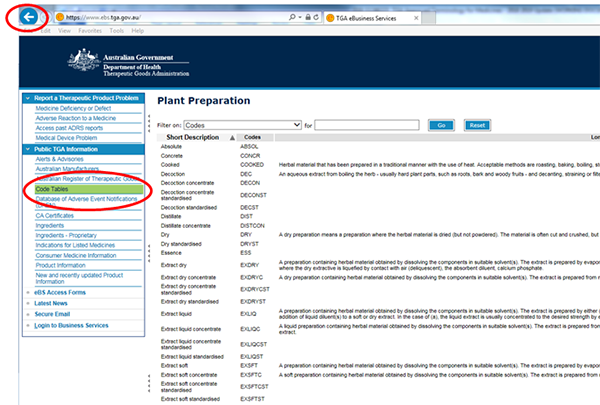
Code Tables
The Code Tables provide terminology for use in applications for registration of therapeutic goods and on product labels.
The Code Tables provide a consistent and standardised method for naming and specifying therapeutic products including: dosage forms, plant preparation etc. See Other terminology for more detail.
For a full list of Code Table terminology, refer to the Code Tables homepage.
Each result will generally list the following:
Short Description - full name of the approved terminology
Codes - terminology abbreviation
Long Description - A description of the approved terminology (where possible)
How to search the Code Tables
- Go to Code Tables.
- Select a specific category, for example, 'plant preparation'.
To navigate back to the Code Tables homepage
- Select the back button in the top left hand corner or
- Go to the left meu and click Code Tables under Public TGA information.