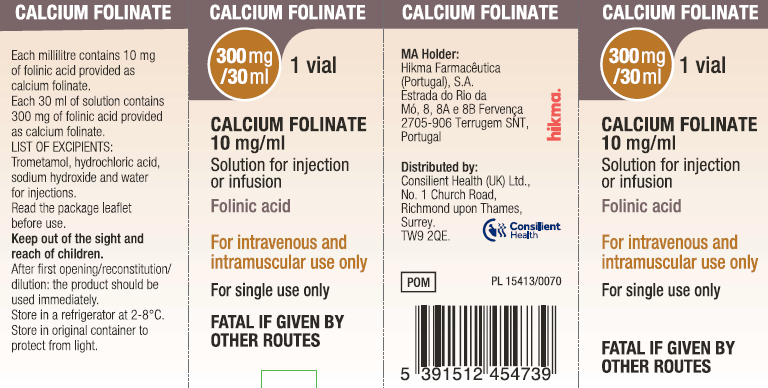
(Approval lapsed) Calcium folinate 300 mg/30ml solution for injection or infusion vial (Hikma/Consilient UK)
Section 19A approved medicine
(Approval lapsed) Calcium folinate 300 mg/30ml solution for injection or infusion vial (Hikma/Consilient UK)
Section 19A approval holder
ORSPEC Pharma Pty Ltd ABN 15 634 980 417
Phone
02 4339 4239
Approved until
Status
Expired
Medicines in short supply/unavailable
DBL LEUCOVORIN CALCIUM folinic acid 300 mg/30 mL (as calcium folinate) injection vial - ARTG 116688
Indication(s)
Reducing the toxicity and circumventing the effect of folic acid antagonists, if therapeutically desired.
Images