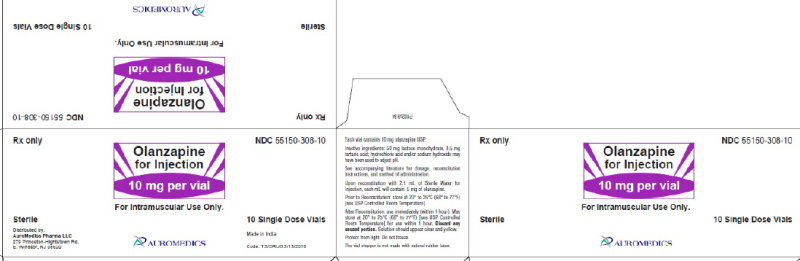
(Approval lapsed) Olanzapine for intramuscular injection 10mg single dose vial (Auromedics)
Section 19A approved medicine
(Approval lapsed) Olanzapine for intramuscular injection 10mg single dose vial (Auromedics)
Section 19A approval holder
Medsurge Healthcare Pty Ltd ABN 92 124 728 892
Phone
1300 788 261
Approved until
Status
Expired
Medicines in short supply/unavailable
ZYPREXA IM olanzapine 10mg powder for injection vial ARTG: 76867
Indication(s)
Rapid control of agitation and disturbed behaviours in patients with schizophrenia and related psychoses and in patients with acute mania associated with Bipolar 1 Disorder, when oral therapy is not appropriate.
Images