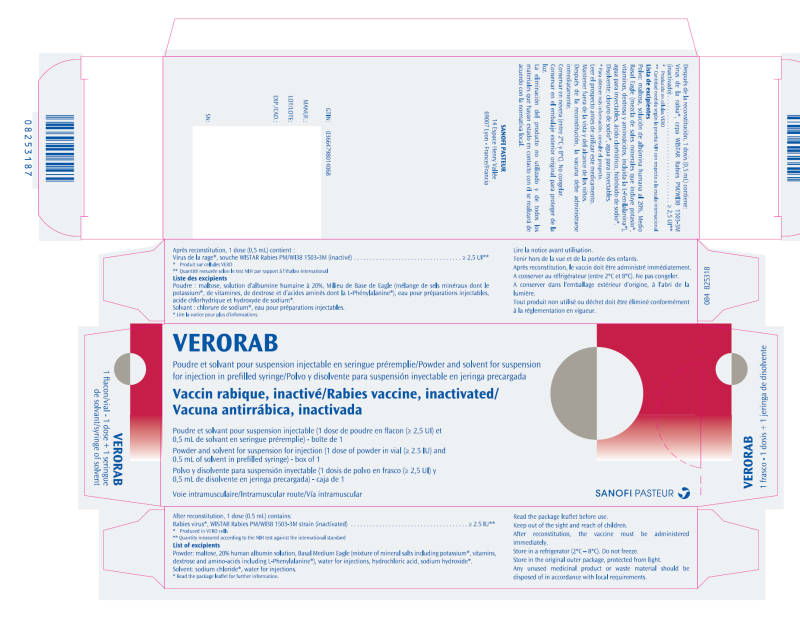
(Approval lapsed) VERORAB (Rabies vaccine 2.5IU) powder and solvent for suspension for injection in prefilled syringe
Section 19A approved medicine
(Approval lapsed) VERORAB (Rabies vaccine 2.5IU) powder and solvent for suspension for injection in prefilled syringe
Section 19A approval holder
Sanofi-Aventis Australia Pty Ltd ABN 31 008 558 807
Phone
1800 818 806
Approved until
Status
Expired
Medicines in short supply/unavailable
MERIEUX INACTIVATED RABIES VACCINE 2.5IU powder for injection vial with diluent syringe - ARTG 26675
Indication(s)
VERORAB is indicated for pre-exposure immunisation in persons at special risk of contracting rabies as well as post exposure immunisation against rabies.
Images