The government is now operating in accordance with the Guidance on Caretaker Conventions, pending the outcome of the 2025 federal election.
A new Scheduling Policy Framework (SPF) and Scheduling Handbook
Following extensive stakeholder, and in collaboration with State and Territory departments, the SPF has been redrafted as two documents - a policy document, the SPF, and a guidance document, the Scheduling Handbook.
The SPF contains the factors for each schedule and appendix, and the new version has been endorsed by the Australian Health Ministers Advisory Council (AHMAC, the committee of heads of State, Territory and Commonwealth Health departments). The SPF remains an AHMAC document and any future changes will require AHMAC consideration and endorsement.
The Scheduling Handbook contains details of the scheduling process, including when and how matters are referred to the Advisory Committees, information on the public consultation process and guidance for applicants. The Handbook is a Department of Health document and will be maintained by the Scheduling Secretariat within the Health Products Regulation Group of the Department. Changes to the Handbook can be made without referral to AHMAC, but will be made in consultation with the Delegates, and/or the Advisory Committees for Medicines and/or Chemicals Scheduling There may also be a public consultation depending on the significance of the change.
Scheduling Working Group
A new "Scheduling Working Group" has been established comprising representatives from the states and territories, the medicines and chemical scheduling advisory committees, industry groups and professionals from the medicine, pharmacy and chemicals sectors as well as consumer representatives.
- AHMAC has agreed that a new appendix in the Poisons Standard would be established to enable additional controls or requirements for Pharmacist-only medicines (Schedule 3 substances) to be specified, in particular those recently down-scheduled from Schedule 4 (Prescription only). The criteria to be covered in the new schedule needs to be considered by the Working Group, to provide advice to the Department.
- The Working Group will develop a framework and guidelines for proactively identifying medicinal substances for consideration for rescheduling of Schedule 4 to Schedule 3 and/or 2, noting that it was agreed any such candidates would need to be considered in the usual manner through the usual committee and delegate processes following submission of an application for rescheduling.
- In the immediate term, the Working Group will provide significant input on potential regulatory controls that may be appropriate for inclusion in legislation around advertising of Schedule 3 (Pharmacist-only) medicines (i.e. within the revised Therapeutic Goods Advertising Code).
The first meeting was held on 9 February 2018 and the following presentation was presented:
Medicines Scheduling and Scheduling Policy Ad Hoc Working Group - Meeting One, February 2018
The second meeting was held on 6 March 2018 and the following presentation was presented:
Medicines Scheduling and Scheduling Policy Ad Hoc Working Group - Meeting Two, March 2018
Interim decision consultation
Public consultation on the interim decisions will now be open to any interested party, and the time frame has been extended to up to 4 weeks, to allow more meaningful submissions to be prepared.
More delegate discretion for referral to the Advisory Committee(s)
The SPF and Handbook now provide the delegate with greater discretion for whether to refer a matter to the Advisory Committee or to make a decision directly.
In most cases, where consideration of a substance is straightforward and fits within the defined cut-offs, the delegate may choose to make a scheduling decision. Similarly, if a substance is a second-in-class, or similar to substances considered previously, the delegate may choose not to refer to the Advisory Committee.
Some of the Appendix considerations will now also be Delegate decisions, rather than referral to the Advisory Committee.
Processes have been developed to ensure stakeholders are kept informed of these decisions.
Other changes following the review
(Note: Further details can be found in the Scheduling Handbook)
- Provision for a new appendix in the Poison Standard to enable additional controls or requirements for Pharmacist-only medicines (Schedule 3 substances) to be specified.
- New process to ensure more timely market access to encourage submission of suitable applications for rescheduling of Schedule 4 medicines to Schedule 3 and/or Schedule 2.
- Applications for chemicals scheduling consideration can be submitted directly to the Secretariat.
- The applicant is now required to submit a summary for publication outlining the scheduling proposal, including the name of the substance, the proposed schedule, and a brief description addressing the relevant scheduling factors.
- Guidance is included in the Scheduling Handbook for applicants to use a published risk:benefit model when preparing their submissions.
- It was agreed to undertake a limited trial to assess the value of applicants presenting to the advisory committees. Further advice will be provided regarding the selection of presenters and the purpose of such presentations, as it would not be appropriate for the presenters to repeat material that is already in their submission nor for new issues to be raised in the presentation that had not been included in the submission.
Schedule 3 Advertising reforms
The default position regarding advertising of Schedule 3 substances has shifted to allow substances to be advertised unless the Scheduling Delegate determines they should not be. To allow substances in Schedule 3 to be advertised to the general public, a decision for each substance will still need to be made by the delegate for inclusion in Appendix H of the Poisons Standard on a case by case basis, regardless of whether they are old or new listings.
The SPF factors for Appendix H have been revised as follows:
Appendix H - Schedule 3 medicines permitted to be advertised
A new or amended entry to Appendix H may be made by the Secretary after taking into account matters set out in the Guidelines for advertising of substances included in Schedule 3 of the Poisons Standard (the Guideline).
Schedule 3 medicine substances will be included in Appendix H unless the Secretary determines there are reasons for not permitting the advertising of a particular substance. The Guideline sets out when substances cannot be advertised.
It is planned that the working group mentioned above will consider the substances currently in Schedule 3 but not in Appendix H for suitability for advertising, and provide advice to the scheduling delegate to inform his/her final decision.
This group will also provide input to the factors for determining suitability for advertising, which will be published in the new "Guidelines for advertising substances included in Schedule 3 of the Poisons Standard". They will also provide input to the mandatory requirements for advertisements for these substances which will be incorporated in the Therapeutic Goods Advertising Code, which will undergo public consultation in early 2018.
Identification of candidate substances for pro-active down-scheduling
Identification of candidates for potential consideration for down-scheduling (‘switching’) from Schedule 4 to Schedule 3 has been considered by the working group. The driver for this change to the SPF is to facilitate better access to medicines and support appropriate self-care. The working group noted that similar stakeholder groups had provided advice of this type to regulators in UK, Ireland, Denmark and Singapore.
It was agreed that while the working group could assess the potential of products for down-scheduling, and identify factors to be considered and how risks could be best mitigated, a formal application to the TGA would be required to initiate consideration of individual substances for rescheduling.
At a meeting of the working group held on 11 December, following a request by the convenor, the Pharmaceutical Society of Australia proposed for discussion a list of priority substances that could be considered for rescheduling (Table 1). The list was developed from a survey sent out to branch presidents and state managers for distribution to local branch committees, as well as to PSA staff pharmacists.
The survey listed 15 medicines or medicine classes, and asked respondents to indicate their support and priority for down-scheduling each.
The list of 15 medicines/classes was developed by reviewing recent down-scheduling decisions and comparing S3/S4 availability internationally, and reviewing all Schedule 4 medicines for potential clinical suitability for pharmacist supply. Respondents were invited to provide free text comments on their selections and suggestions for additional medicines/medicine classes.
211 responses were received from all states and territories. The primary practice setting of the major groups of respondents was as follows: community pharmacy 73%, hospital pharmacy 19%, professional organisation 9%, academia 7% and consultancy 5%. Figure 1 below shows the relative priorities for down-scheduling as expressed by the surveyed group:
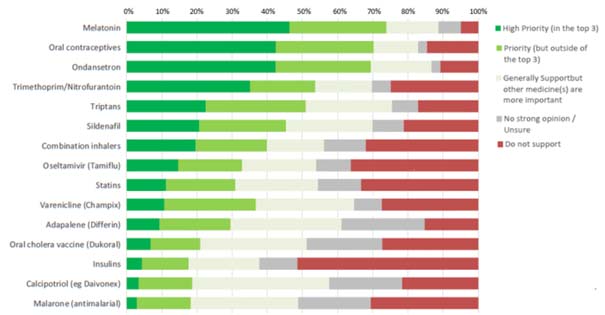
The responses provide a range of reasons for and against the down-scheduling of particular substances. In some cases, respondents proposed that alternative options for supply for pharmacists be considered such as the use of continued dispensing provisions, or potential supply through locally-developed (i.e. State and Territory health department) authorisations through an approved treatment pathway. In discussions to date, seven key substances, or groups of substances, were identified by the PSA as high priority for pro-active down scheduling (see Table 1).
| Substances | Pros | Cons |
|---|---|---|
| Triptans | Commonly available as S3 in other countries. Need for early initiation of treatment provides significant clinical support for pharmacist supply. | None identified |
| Melatonin | Safety factor, particularly when compare against existing S3 options. | Some registered products are scheduled as prescription medicines in many countries. |
| Ondansetron | Positive safety factor. Good clinical indication, particularly if have strong pathway for use. | Does not appear to be OTC in any other country |
| Oral Contraceptive Pills | Useful consumer benefit. Limited rescheduling in other countries. | Likely requires strong caveats and controls around when pharmacists can / cannot supply. |
| Trimethoprim | Very favorable consumer benefit. Clinical pathway/protocol to guide use required | E.coli antimicrobial resistance is growing. Would requires strong compliance with treatment protocol and need to consider current antimicrobial stewardship emphasis in health systems |
| Adapalene | Relatively safe, simple diagnosis. Other substances may have a higher priority. | Potential opposition with respect to appropriate diagnosis/misuse. |
| Sildenafil | Interest remains |
An additional list of substances that are S4 in Australia, but already available without prescription in some form in at least one comparable international jurisdiction was prepared by the Australian Self-Medication Industry (ASMI, Table 2) at the request of the convenor. Some of the medicines shown in Table 2 may have additional conditions, such as record keeping and/or pharmacist consultation and other limits on supply, depending on the jurisdiction. Appendix M may provide a suitable, potentially more nationally aligned mechanism for comparable conditions or controls.
| Category/Actives | Indication | OTC in other jurisdictions |
|---|---|---|
| Adapalene | Acne - Retinoid analogue | NZ, US |
| Sildenafil | Erectile Dysfunction | NZ, UK |
| Oseltamivir | Influenza | NZ |
| Sumitriptan | Migraine | NZ, UK, Sweden |
| Rizatriptan | Migraine | NZ, Sweden |
| Trimethoprim | Uncomplicated urinary tract infections | NZ |
| Statins | Cholesterol lowering agent | UK |
| Tamsulosin | Benign prostatic hypertrophy | UK |
| Estriol pessaries | Menopause symptoms | Sweden, Denmark, Finland, Hungary, Norway |
| Oral contraceptives | Birth control (continued supply) | NZ |
| Azithromycin | Chlamydia | UK |
| Oxybutynin | Overactive bladder (female) | US |
| Tranexamic acid | Menorrhagia | UK, Sweden |
| Proguanil | Malaria prophylaxis | UK |
| Mupirocin | Topical antibacterial | Canada, Singapore, South Korea |
| Metronidazole (topical) | Rosacea | Sweden, Norway, Italy, Finland, China |
| Calcipotriol | Psoriasis | NZ, UK, Ireland |
| Melatonin | Sleep/Insomnia | NZ, Canada, US |



