Consumers and health professionals are advised that Medtronic Australasia, in consultation with the TGA, has issued a hazard alert for two models of Medtronic Neuromodulation Deep Brain Stimulation (DBS) system pocket adaptors (model numbers 64001 and 64002).
Medtronic Neuromodulation DBS system pocket adaptors are used with Activa PC (model 37601) and Activa RC (model 37612) neurostimulators when these models are used to replace older generation DBS devices without having to replace the already-implanted extensions and leads. DBS devices are implantable, programmable medical devices that deliver electrical stimulation to the patient’s brain. These devices are used to treat the symptoms associated with movement disorders, epilepsy and Parkinson’s disease, as well as other conditions.
Globally, it has been identified that 16 Medtronic Neuromodulation DBS system pocket adaptors have been returned due to high impedance, resulting in decreased current being conducted through the device. Some of the reported cases involved loss of therapy, return of symptoms, rebound effects and potential revision surgery to replace the device. There have been no incidents reported in Australia.
Analysis of the returned devices found the cause of the high impedance to be conductor wire fractures near where the wire exits the neurostimulator connector block. In two cases, the issue was identified during implantation surgery, while the other 14 were identified after implantation and resulted in revision surgery.
Information for consumers
If you or someone you provide care for has an Activa PC or Activa RC neurostimulator that uses a Medtronic Neuromodulation DBS system pocket adaptor, be aware of this issue.
If you suspect a return of underlying disease symptoms or any other unexpected problems, contact your managing physician.
If you have any questions or concerns about this issue, talk to your health professional.
Information for health professionals
If you are treating a patient who has an Activa PC or Activa RC neurostimulator that uses a Medtronic Neuromodulation DBS system pocket adaptor, be aware of this issue.
If they present with signs of potential loss of therapy, refer them to their managing physician.
Information for managing physicians and surgeons
If you are managing the treatment a patient who has an Activa PC or Activa RC neurostimulator that uses a Medtronic Neuromodulation DBS system pocket adaptor, be aware of this issue.
Consider advising these patients of this issue and educating them regarding signs that their device may be affected by high impedance. Instruct them to contact you if any problems are suspected.
In some cases, a loss of DBS therapy may cause significant or life threatening rebound effects, including status dystonicus in the dystonia patient population; rebound effects, which in rare cases may constitute a medical emergency in the Parkinson’s disease population; and status epilepticus in the epilepsy patient population.
Patients receiving DBS for essential tremor may experience a return of symptoms that may be of greater intensity, which could constitute a medical emergency.
Patients being treated for obsessive compulsive disorder (OCD) may have a recurrence of physical and psychological OCD symptoms, including anxiety and depression, with the potential for greater intensity.
During implantation
The current labelling for the handling of Medtronic Neuromodulation DBS system pocket adaptors during implantation is described within the device manual.
The instructions in the below excerpt from the DBS pocket adaptor implant manual will help minimise potential for conductor wire fracture by ensuring that the wire is not bent sharply or kinked at the time of implant.
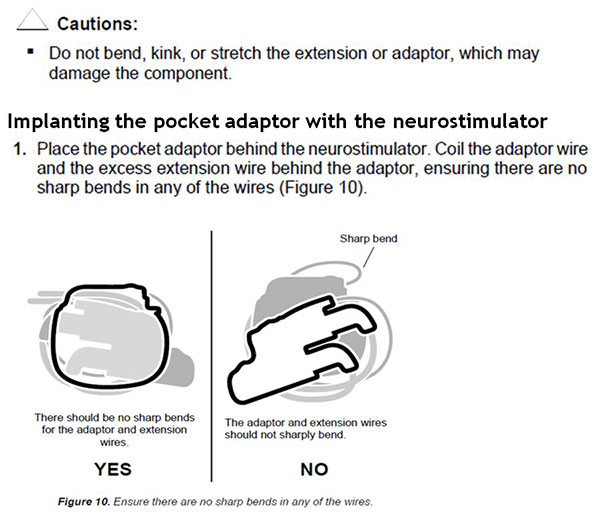
In addition, you should complete a system integrity check for proper electrode impedances before pocket closure, as described in the excerpt from the Activa PC Model 37601 implant manual below.

If you have any questions or concerns about this issue, contact Medtronic Australasia on 1800 668 670.
Reporting problems
Consumers and health professionals are encouraged to report problems with medical devices. Your report will contribute to the TGA's monitoring of these products. For more information see the TGA Incident Reporting and Investigation Scheme (IRIS).
The TGA cannot give advice about an individual's medical condition. You are strongly encouraged to talk with a health professional if you are concerned about a possible adverse event associated with a medical device.

