Consumers and health professionals are advised that Synthes Australia, in consultation with the TGA, is issuing a hazard alert for its BC Distractor Body, a component of the Craniomaxillofacial (CMF) Distraction System.
Synthes Australia is also recalling unused stocks supplied to hospitals of the affected devices.
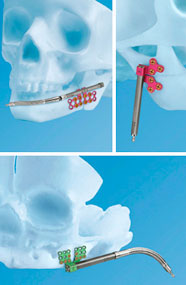
The CMF Distraction System comprises a number of modular components, which together are implanted in patients to stabilise or incrementally lengthen parts of the jawbone. It is used to correct congenital deficiencies or defects resulting from trauma.
It has been identified that in rare circumstances an implanted BC Distractor Body may reverse after surgery. This means the extent of the lengthening could reduce to less than the desired length.
If this occurs, the patient may experience complications, including poor joint mechanics and pain, and require additional surgical procedures. There is potential for patients with an already compromised jaw anatomy to suffer breathing difficulties.
There have been 10 reports worldwide that could be related to this issue.
The affected components are:
| Product description | Part number | Lot numbers |
|---|---|---|
| BC Distractor Body, end activated, distraction length 15 mm, with Universal Joint, for CMF Distractor | 04.315.063 | 6883301 |
| BC Distractor Body, end activated, distraction length 30 mm, with Universal Joint, for CMF Distractor | 04.315.066 | 7082908 |
| BC Distractor Body, end activated, distraction length 40 mm, with Universal Joint, for CMF Distractor | 04.315.068 | 7266969, IS10386 |
Information for consumers
Synthes Australia has written to surgeons who have implanted CMF Distraction Systems using BC Distractor Bodies and hospitals where these operations were performed, providing further information about this issue, including advice on how to treat affected patients.
Synthes Australia is not recommending pre-emptive replacement of implanted devices that are not showing signs of this issue.
If you or someone you care for has a CMF Distraction System and you have any questions or concerns about this issue, contact your health professional or the hospital where the operation was performed.
The likelihood of patients experiencing problems as a result of this issue is low. However, if you or someone you care for who has a CMF Distraction System experiences any unusual symptoms such as pain or breathing difficulties, promptly contact your health professional or the hospital where the operation was performed.
Information for all health professionals
If you are treating a patient who has a CMF Distraction System, and they or their caregivers have any questions or concerns about the above issues, refer them to their managing surgeon or the hospital where the operation was performed.
Reassure them that the likelihood of patients experiencing problems associated with this issue is low.
Advise them that, if they experience any unusual symptoms such as pain or breathing difficulties or symptoms suggestive of a change in temporomandibular joint mechanics, to promptly contact their managing surgeon or the hospital where the operation was performed.
Information for surgeons and hospitals
Synthes Australia has written to surgeons who have implanted CMF Distraction Systems using BC Distractor Bodies and hospitals where these operations were performed, providing further information about this issue, including advice on how to treat affected patients and the recall process for unused stock.
Patients who have received a CMF Distraction System using a BC Distractor Body should be closely monitored until a clinical and/or imaging examination confirms the desired outcome.
The type of post-operative care and support treatment should be determined based on the individual patient's circumstances.
When speaking to patients and caregivers about this issue, reassure them that the likelihood of them experiencing problems associated with this issue is low.
Advise them that, if they experience any unusual symptoms, including poor joint mechanics, pain or breathing difficulties, to promptly contact you or the hospital where the operation was performed.
Reporting problems
Consumers and health professionals are encouraged to report problems with medical devices. Your report will contribute to the TGA's monitoring of these products. For more information see the TGA Incident Reporting and Investigation Scheme (IRIS).
The TGA cannot give advice about an individual's medical condition. You are strongly encouraged to talk with a health professional if you are concerned about a possible adverse event associated with a medical device.

