Consumers and health professionals are advised that Johnson & Johnson Medical, in consultation with the TGA, has issued a hazard alert for all lots of its Limb Preservation System (LPS) lower extremity dovetail intercalary component.
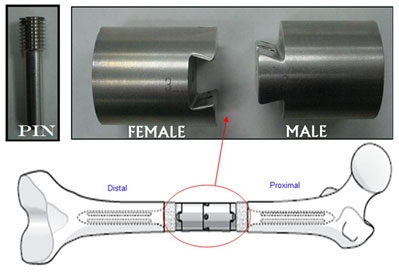
The LPS lower extremity dovetail intercalary component is used as a replacement for the mid-shaft portion of a femur after it has been surgically removed as a result of a tumour, infection, trauma, or for some other medical reason.
It has been identified that there is potential for the female component of the device to break in some circumstances.
Testing of the device has been undertaken, simulating loads under normal walking conditions.
The following patient conditions or activities can increase the force exerted on the device and may result in a higher risk of breakage:
- weighing more than 90 kg, with the risk increasing as weight increases
- walking up and down stairs
- going from sitting to standing.
Other factors, including the patient's musculature, activity level and overall health, are also important considerations.
Information for consumers
Johnson & Johnson Medical has written to surgeons who have implanted LPS lower extremity dovetail intercalary components, providing further information about the above issue and advice on how to treat affected patients.
If you have had an LPS lower extremity dovetail intercalary component implanted and experience unexpected pain or other unusual symptoms (for example, loss of leg function), or if you have any questions or concerns about the above issue, you should contact your surgeon or the hospital where the surgery was undertaken.
Information for all health professionals
Patients who have had an LPS lower extremity dovetail intercalary component implanted and who have any questions or concerns about the above issue should be referred to their surgeon or the hospital where the surgery was undertaken.
Information for orthopaedic surgeons
Johnson & Johnson Medical has written to surgeons who have implanted LPS lower extremity dovetail intercalary components, providing further information about the above issue and advice on how to treat affected patients.
Prophylactic revision in the absence of symptoms is not recommended at this time. However, surgeons should consider speaking with patients who have already received an LPS lower extremity dovetail intercalary component about the risks of implant failure and how to detect it if the patient begins experiencing symptoms. This advice will differ depending on the patient's weight and activity level.
The manufacturer is investigating design options to address this issue in the future. In the meantime, the device will only be available for specific revision cases. Surgeons should consider each patient’s individual circumstances when deciding on the best treatment options.
Additional information
Possible clinical implications if an LPS lower extremity dovetail intercalary component fractures, include:
- poor mechanics and loss of function
- pain
- separation of the male component from the female component
- adverse tissue reaction if a piece of the female component completely breaks free and causes irritation to the surrounding tissue
- damage to bone - surgeon may have difficulty undertaking revision surgery due to lack of sufficient bone stock.
Reporting problems
Consumers and health professionals are encouraged to report problems with medical devices. Your report will contribute to the TGA's monitoring of these products. For more information see the TGA Incident Reporting and Investigation Scheme (IRIS).
The TGA cannot give advice about an individual's medical condition. You are strongly encouraged to talk with a health professional if you are concerned about a possible adverse event associated with a medical device.

