On this page: Introduction | Major issues raised by the Stokes Review | Conclusion
Introduction
The Australian public, rightly, expects health authorities to have effective mechanisms in place to manage safety issues that might arise with vaccination programs. They also wish to be adequately informed about the nature of the vaccine they are receiving. It is hoped that this response to the Stokes Review will enable a greater public understanding of the nature of the controls that are in place and that do work effectively to protect public health in Australia.
On 11 August 2010, the WA Health Minister tabled in State Parliament a report of a review conducted by a former senior WA Health Official, Professor Bryant Stokes, into the handling of the suspension of the 2010 WA seasonal influenza vaccination program for children.
The TGA has not responded publicly to the review provided to the WA government. However, the ongoing discussion on the issue is continuing to damage public confidence in what is objectively one of the world's best vaccine programs. In addition, the WA Department of Health has announced that it wishes to take the recommendations of the Stokes Review to the Australian Health Ministers' Conference. It is important that the facts about the investigation into the events leading up to the suspension and an accurate understanding of the existing Commonwealth and State and Territory mechanisms for monitoring vaccine safety are in the public domain.
As Professor Stokes notes in his report, "sadly, public perception of vaccination programs has been damaged by these events and it will take time to reverse this view". Professor Stokes' report (the Stokes Review) makes a number of observations and recommendations that demonstrate a lack of understanding of the existing frameworks that operate in Australia and internationally to ensure the safe delivery of vaccine programs. This in turn has led to the formulation of some recommendations which may further serve to damage public perception of vaccine programs.
The Stokes Review cites six major areas of criticism regarding the handling of the investigation and management of the adverse events associated with the 2010 seasonal flu vaccine program:
- Perceived deficiencies in current reporting mechanisms for adverse events,
- A lack of monitoring of vaccine safety,
- A slow response to the issue by relevant authorities,
- A lack of information about vaccines for consumers,
- Perceived conflicts of interest in the TGA's role and its funding,
- Problems within the WA Department of Health reporting and governance arrangements.
As will be outlined in this document, the Stokes Review contains a number of fundamental misunderstandings that have led to erroneous and often ill-founded conclusions. These matters were raised with the reviewer prior to the tabling of the report in the WA Parliament. Importantly, the review did not examine the documentation held by the TGA that establishes the facts of the TGA's investigation. Moreover it appears that the review did not adequately verify its understanding of the existing framework in place in Australia for monitoring vaccines, nor its interpretation of relevant international comparisons. As a result, the Stokes Review draws a number of inferences about the Commonwealth's handling of this issue that are not supported by the facts.
Major issues raised by the Stokes Review
1. Perceived deficiencies in current reporting mechanisms for adverse events
Australia has one of the highest per capita rates of reporting of medicines adverse events in the world, not because we are more prone to side effects, but rather because we have one of the most effective adverse event reporting frameworks in the world.
This system of spontaneous reporting of adverse events was developed in the early 1970s and has served Australia well over the past four decades to allow early identification of adverse events that may require urgent regulatory action. In recent years, amongst other things, this system of spontaneous reporting has allowed the clinical and scientific experts at the TGA to identify a number of safety problems that have resulted in regulatory action before any other part of the world. For example, the spontaneous reporting system identified liver failure secondary to lumiracoxib (Prexige) which resulted in the worldwide recall of the medicine following the TGA's initial action to remove it from the market in Australia.
The spontaneous reporting system, which initially relied on reporting by doctors only, has been expanded over the years to encourage reporting of adverse events from other healthcare professionals and directly from the general public. The system is a voluntary reporting system that encourages anyone who believes they have suffered an adverse event to a medicine or vaccine to contact the TGA with details of the adverse event so that it can be entered into the TGA's adverse event database. Extensive information is available on the TGA website (www.tga.gov.au) explaining how to report a suspected adverse event regarding a medicine, and the nature of the information required.
Reports may be provided in writing to the TGA
- By mail to: Medicines Safety Monitoring, Reply Paid 100, WODEN ACT 2606
- By fax to: 02 6203 1616
- By email to: adr.reports@tga.gov.au
- Online at: https://www.ebs.tga.gov.au/ebs/ADRS/ADRSRepo.nsf?OpenDatabase
In addition to these mechanisms consumers may also report adverse events to a Commonwealth-funded Consumer Adverse Medicines Event line. The TGA website advises:
Report an adverse reaction to a medicine
Consumer Adverse Medicine Events Line: Ph 1300 134 237
This phone-in service, provided by the Mater Hospital, Brisbane, is available for members of the general public who suspect they have experienced an adverse medicine event. The service forwards reports of suspected adverse reactions to the TGA.
The Consumer Adverse Medicines Event Line is funded by the National Prescribing Service and provides consumers with a mechanism to report adverse experiences with medicines and an opportunity for consumers to consult with a pharmacist about medicine safety.
As well as voluntary reporting mechanisms for consumers and healthcare professionals, it is mandatory for companies that supply medicines in Australia to report adverse events to the TGA within prescribed timelines.
The TGA provides a "one-stop" access point for reporting adverse events to any medicine or vaccine for consumers, healthcare professionals, the States and Territories and medicines companies. In the past twelve months the TGA has received approximately eighteen thousand individual reports of adverse reactions to medicines or vaccines.
Once reports are received by the TGA, they undergo classification and clinical review and are entered into the national adverse event database to allow the TGA's Office of Product Review to identify whether a particular medicine is associated with an unusual or unexpected rate of adverse events.
It is important to note that there are a number of limitations to the use of numbers of adverse events as the sole or primary mechanism to determine whether a medicine or vaccine is safe. The Stokes Review implies that a numerical analysis of the number of adverse event reports divided by the number of doses of vaccine administered will allow immediate determination of the safety of the vaccine. This reflects an apparent lack of understanding of the need to carefully analyse each case report to determine whether it actually is the type of clinical event that it has been reported as, and whether it has any relationship to the administration of the vaccine.
For example an adverse event report of a "convulsion" may be a febrile convulsion, an epileptic seizure, a post-fainting fit, a stroke or a variety of other clinical conditions. Careful clinical review is required to determine the likely significance and any causal relationship that might exist between the clinical event and the vaccine. If the incorrect condition is included in the database, subsequent numerical analysis of the significance of the data will be impaired. Similarly, the likelihood that the clinical event was due to the vaccine needs to be carefully considered. Questions such as: "Did the clinical event occur before or after the vaccine was administered?" "Was there a previous history of the same clinical event unrelated to administration of the vaccine?" "Were there other factors in this individual that might be relevant, such as the use of other medicines, the presence of other illnesses etc?" all need to be carefully considered.
This clinical review of adverse event reports is the foundation upon which any investigation of a major safety signal rests. All adverse event reports for medicines and vaccines are reviewed by clinical staff at the TGA and, where necessary, additional clinical information is sought from the reporter of the adverse event.
The TGA utilises the clinical and scientific expertise of the staff of the Office of Product Review and the advice of clinical experts on its statutory and non-statutory advisory committees to interpret the clinical significance of reports received through the spontaneous reporting system. In the case of a major public health investigation such as the TGA undertook in relation to the 2010 seasonal influenza vaccine, the TGA may also establish specific additional expert advisory committees to assist in the investigation. For this investigation the TGA established an epidemiological expert group to provide advice on the analysis of existing state and national datasets that might inform the analysis of the significance of the adverse events. The TGA also established a scientific advisory panel made up of Australia's leading scientific experts to work with the TGA's laboratory experts and advise on types of laboratory tests that could help identify the possible cause of the adverse events.
In some States and Territories it is also mandatory for vaccine providers to report adverse events to their respective health departments. The TGA has established cooperative arrangements with all States and Territories that ensure that reports sent to States and Territories are forwarded to the TGA for inclusion in the adverse event database to enable further analysis. Similarly, the TGA provides a monthly report to each State and Territory to advise it of all the vaccine adverse event reports the TGA has received to ensure that the States and Territories are aware of events occurring in their jurisdiction. This cooperative arrangement has enabled the creation of safeguards within the reporting framework that ensure that vaccine safety issues arising in one state are reported nationally and that appropriate analysis and action can be coordinated nationally by the TGA. This framework has been endorsed on several occasions by all States and Territories.
Contrary to the conclusion of the Stokes Review, the ability to have all vaccine adverse events reported to the national adverse event database at the TGA, and also provided to and from States and Territories, is one of the strengths of the Australian vaccine safety monitoring framework. Although, there may certainly be room for improvement in the timeliness with which the Communicable Disease Control Directorate of the WA Department of Health provided reports to the TGA arising from their state-based trial program, in general this system has functioned effectively to monitor the national immunisation program.
An additional mechanism of capturing vaccine reporting data, known as the Australian Childhood Immunisation Register, has been established by the Commonwealth. This data system is managed by Medicare Australia and records the numbers of doses of vaccines provided to children under the National Immunisation Program. The TGA is able to access information collected by the ACIR to assist in its analysis of the significance of adverse events but, contrary to what is stated in the Stokes Review, does not directly fund, manage or have responsibility for the ACIR.
It is important to note that the ACIR database, which relies on electronic reporting by general practitioners when they administer a vaccine, was not designed to monitor vaccine programs outside the National Immunisation Program. It was not established, nor is it able to act as a suitable monitoring program, for the WA Department of Health's childhood influenza immunisation program. That Department remains responsible for ensuring there is effective monitoring and adverse event reporting programs in place for clinical trial programs they wish to establish outside the National Immunisation Program.
Information about adverse reactions to medicines is currently available in the form of summary reports and case summary reports on request from the Office of Product Review (OPR) at the TGA. Requests can be made by email to adr.reports@tga.gov.au or by ringing the OPR enquiries line on 1800 044 114.
In providing information about adverse event reports great care is taken to remove any private or personal information from the report that may be used to identify the individual who is the subject of the report.
The current reporting arrangements for adverse events related to vaccine use are appropriate, allow national collation of data and close cooperation between Commonwealth and State and Territory health departments. The Stokes Review recommends improvements in the timeliness of reporting to the TGA by the WA Communicable Disease Control Directorate, and this recommendation is supported by the TGA.
The Stokes Review highlights the need to raise levels of community awareness of the mechanisms available to it to report adverse events, and the TGA is currently developing a strategy to improve understanding of available reporting mechanisms. The TGA will work with consumers, health professionals and organisations, and other Commonwealth and State and Territory health authorities to implement this program.
The Stokes Review did not address the matter of what reporting arrangements the WA Department of Health had put in place for its state-based cohort program of childhood influenza vaccination.
2. A perceived lack of monitoring of vaccine safety
The spontaneous adverse event reporting system described above is just one of the mechanisms the Commonwealth and State and Territory departments of health have established to monitor the safety of vaccines. Vaccine manufacturers are inspected for the quality of their production facilities and must comply with international standards that are rigorously assessed through regular programs of audit by the TGA and its international regulatory counterparts. The vaccines themselves undergo a rigorous pre-market evaluation that the TGA applies to all prescription medicines and which is consistent with all major medicines regulatory authorities around the world. Since 2009, the TGA has required companies wishing to register new medicines and vaccines to submit risk management plans to identify how they will manage the emergence of any safety issues arising when their vaccine is released to the market. The TGA oversees the release of vaccine batches and does pre-release batch testing of influenza vaccines to ensure that they meet the required standards of purity and potency. The Commonwealth funds the National Centre for Immunisation Research and Surveillance (NCIRS) to undertake epidemiological analysis to evaluate the effects of vaccine programs around the country, and there are a number of specific advisory committees containing scientific and clinical experts that guide the design, implementation and oversight of the National Immunisation Program.
The Stokes Review claims that although the World Health Organization (WHO) had advised all countries administering pandemic vaccines to conduct intensive monitoring for safety and efficacy, this was not done in Australia. This is incorrect.
The WHO advice was developed to guide countries on how to monitor the rollout of pandemic, not seasonal influenza vaccines. The advice was provided by WHO in the expectation that in the event of a pandemic, doses of pandemic vaccine would be administered to millions of people in a very short period of time. This advice was prudent, and aimed at ensuring that any safety problems could be detected and responded to quickly. Australia established monitoring processes for the rollout of the Panvax pandemic vaccine in accordance with the WHO guidelines and had an active surveillance program in place involving State, Territory and Commonwealth health authorities throughout the implementation of the pandemic vaccine program.
The WHO advice was not developed to guide the monitoring of the routine use of seasonal influenza vaccine within national immunisation programs. Seasonal flu vaccine has been available on an annual basis for decades and its safety profile has been well characterised through its effective use in millions of people over many years. The vaccine safety reporting and monitoring framework described above has been designed to effectively identify and respond to safety signals emerging from the much more gradual use of influenza vaccine that occurs with the usual seasonal vaccine program under the national immunisation program.
The rationale applied by the WHO in their pandemic guidance relates to the need to be able to monitor and respond rapidly when administering doses of vaccine rapidly to a large number of people. These guidelines may well be relevant to considerations by the WA Department of Health regarding appropriate future monitoring arrangements should they wish to continue to undertake mass childhood influenza vaccination outside the national immunisation guidelines.
3. A slow response to the issue by relevant authorities
The Stokes Review correctly notes that "there is evidence that the Communicable Disease Control Directorate of the WA Department of Health were informed of a significant rise in Adverse Events Following Immunisation (AEFIs) in early April 2010 but did not take any further action whilst they gathered data to carefully analyse the situation."
The TGA is unable to comment on the appropriateness of activities undertaken by the CDCD in the three weeks between when it was first notified of adverse events and the provision of case reports to the TGA. The TGA notes, however, that the sooner case reports and supporting clinical material are provided to the TGA, the sooner its expert pharmacovigilance staff and expert advisory committees can make an assessment of the significance of a potential safety signal.
The Stokes Review states that "there was a slow response by the Commonwealth to apparent emerging adverse events arising from the 2010 vaccination program."
This statement is not borne out by the factual timeline outlined below. In fact, the TGA and the other parts of the Commonwealth's vaccine monitoring framework reacted quickly and effectively to reports of safety problems arising within WA's trial influenza vaccine program. The Commonwealth authorities involved in vaccine safety monitoring were able to act effectively even when adverse events arose in a unique state-based population cohort trial of a vaccine used outside national immunisation guidelines, and in the absence of a state- specific monitoring program for the cohort trial.
Timeline of events:
- On 19 March 2010 the WA childhood influenza vaccine program commenced.
- Between 31 March and 13 April 2010 WA Health authorities were advised on several occasions by clinicians and public health officials of side effects, particularly febrile reactions, associated with the 2010 seasonal influenza vaccine. The TGA was not notified by the WA Department of Health at that time, nor were all the individual adverse reactions reported to the TGA.
- On 13 April 2010 the Communicable Disease Control Directorate (CDCD) of WA Health contacted the TGA by email advising that it was receiving reports of side effects with the influenza vaccine and asking if other jurisdictions were experiencing the same.
- By 13 April 2010 the TGA had received only a few reports of adverse events associated with 2010 seasonal influenza vaccine including 4 reports of febrile convulsions (only one from WA), consistent with previous experience and had no indication of an emerging safety signal. The WA Department of Health was advised of this and the TGA requested that WA immediately provide any adverse event reports to the TGA as soon as possible.
- On 14 April 2010 the WA CDCD undertook in writing to provide information about these adverse events to the TGA as it became available but did not send the documentation of any adverse events.
- On 15 April 2010 the TGA repeated its request for details of documented adverse event reports from the WA Department of Health.
- On 16 April 2010, the TGA wrote to all States and Territories asking them to also expedite the submission of reports of adverse events following immunisation with seasonal flu vaccine to the TGA.
- On 20 April 2010 the TGA finally received documentation from WA which consisted of a number of AEFI reports of suspected febrile convulsions in children following receipt of influenza vaccine that appeared to have been collated and held by the WA Department of Health officials for transmission to the TGA as a single batch.
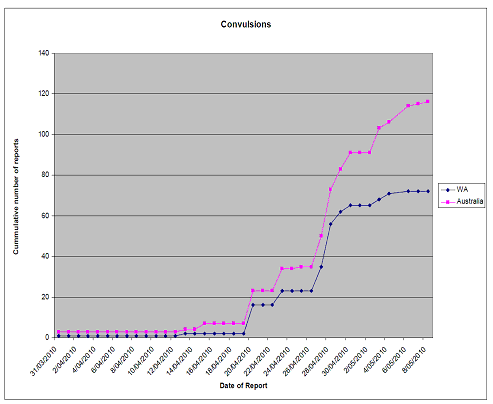
- The TGA presented details of these AEFIs to the National Immunisation Committee (NIC) on 22 April 2010 and again urged all States and Territories to expedite submission of AEFI reports to the TGA. The timeline of reports of febrile convulsions received by the TGA is shown in Figure 1.
- On 23 April 2010 the suspension of the use of the seasonal influenza vaccine in children 5 years and under was announced by the Commonwealth Chief Medical Officer.
The graph in Figure 1 shows the date of receipt of reports of febrile convulsions at the TGA. As can be clearly seen, up until 20 April, the TGA had not received sufficient numbers of clinical reports of adverse events from WA to demonstrate any safety signal with the influenza vaccine.
Within 72 hours of the TGA finally receiving documentation, in the form of adverse event reports it had been actively seeking from WA, the TGA and the Commonwealth Department of Health and Ageing had responded to the issue, with the Australian Government's Chief Medical Officer suspending the immunisation program in children 5 years and under.
Media commentary following the release of the Stokes Review has also questioned why, following the suspension of the use of childhood influenza vaccine, the Commonwealth waited several weeks before announcing that the issue was related to the use of one type of vaccine (Fluvax) and not related to the other influenza vaccines that were available.
In order to ensure that no more children were unnecessarily exposed to the risk of febrile convulsions, the Commonwealth took the precautionary approach of suspending the use of all seasonal influenza vaccines in children under 5 years until an investigation could determine that they were safe. While the investigation has subsequently confirmed that the febrile convulsions were related to Fluvax, at the time the use of the vaccines was suspended (23 April) there was no data available to demonstrate that this problem was confined to only one vaccine. The Commonwealth, rightly, placed the interests of safety ahead of the desire of some vaccine providers to continue their vaccine program utilising other vaccines.
Figure 1
Cumulative reports of convulsion to 7 May 2010
Indeed, as part of the investigation of the Fluvax adverse events it has become clear that febrile convulsions occur with other influenza vaccines, although at rates about 50 times less than for Fluvax, and within the expected range of this side effect reported in the Product Information for the vaccines. The TGA's analysis of the rates of febrile convulsions for the other influenza vaccines has been published on its website: Investigation into febrile reactions in young children following 2010 seasonal trivalent influenza vaccination and Suspected adverse reactions to Panvax® reported to the TGA.
The Stokes Review did not examine the reasons why the WA Department of Health authorities did not report adverse events to the TGA in a timely manner, although it is likely that they were attempting to deal in the best way they knew how with a rapidly evolving safety situation within their unique cohort trial vaccination program. The lack of pre established reporting and monitoring processes within the WA vaccine trial program may well have resulted in difficulties in WA health officials being able to cope with the volume of reports they were receiving and resulting in delays in their reporting of case details nationally and their commencement of any form of clinical review of the cases reported to them.
The Stokes Review did not seek to ascertain the dates of provision of adverse event reports to the TGA, and chose not to make this information available in the document tabled in the WA Parliament even though these data had been provided to it by Commonwealth authorities prior to tabling.
4. A perceived lack of available information about the influenza vaccine for consumers
In general, there are several mechanisms by which consumers may receive information about vaccines and other medicines. They may have a conversation during a professional consultation or be given an information sheet by their doctor or vaccine provider; they may receive Consumer Medicine Information (CMI) from their pharmacist; they may seek that information from the TGA website (http://www.tga.gov.au ); they may get information from other government information services such as the National Prescribing Service (http://www.nps.org.au); or they may obtain this from other public information sources.
An important part of any decision to use a vaccine in any child is an appropriate informed consent process that allows the doctor or other vaccine provider and the parents of the child receiving the vaccine to understand the potential risks and benefits of the vaccine. Although vaccination is usually a safe and beneficial healthcare intervention, all medicines have the potential to cause side effects in some people, and it is important to consider these alongside the risks of the disease the vaccine will prevent. This is best done through a conversation specifically addressing these issues with the treating doctor or other vaccine provider prior to vaccination.
More rigorous requirements for informed consent usually apply where a medicine or vaccine is used as part of a formal clinical trial, and may be warranted where a vaccine is being utilised outside national guidelines, as occurred in the WA childhood influenza vaccine program.
The TGA is responsible for approving appropriate Product Information to assist doctors, pharmacists and other vaccine providers with information that will enable them to have an appropriate discussion about the risks and benefits of the use of the vaccine. The Product Information (PI) or CMI is not meant to be a substitute for the advice provided to parents by the treating healthcare professional, rather it should facilitate the provision of full informed consent.
In the past 12 months, in recognition of the need for consumers to have access to accurate information about their medicines, the TGA has begun publishing both PI and CMI on its website (http://www.tga.gov.au/meds/picmi.htm). This is part of a significant process of organisational reform at the TGA aimed at making its regulatory processes more transparent and readily understood.
The Stokes Review incorrectly states that the CMI for patients for CSL's vaccine does not mention the side effects of vomiting and diarrhoea, although these are in the Product Information for professionals. In fact, the approved PI for Fluvax contains extensive information about vaccine content, testing, and safety, and the CMI refers to nausea, vomiting and diarrhoea as recognised side effects. (See Attachment A which may also be obtained from the TGA website at https://www.ebs.tga.gov.au/ebs/home.nsf/ghpr?openAgent&p=PICMISearch.)
Although the TGA may have wishes to the contrary, it is acknowledged that not all consumers will have detailed conversations with their doctors or vaccine providers prior to receiving their vaccine. The TGA has sought to provide an additional avenue for consumers to obtain this information through provision of CMIs on the TGA website. This cannot act as a substitute for appropriate informed consent.
The Stokes Review did not address the question of whether appropriate informed consent had been provided to the families in WA who participated in the WA childhood influenza program where the vaccine was being utilised outside the National Immunisation Program (NIP).
5. Perceived conflicts of interest in the TGA's role and funding
Medicines regulators around the world are responsible for both the initial evaluation of medicines and vaccines, and for the monitoring and regulatory oversight of those medicines and vaccines once they are on the market. This is the situation that applies in Australia, New Zealand, Canada, the United States, throughout Europe, in Japan, China and throughout Asia.
There are good reasons why it makes sense to have the organisation that has the scientific experts who have evaluated all the toxicology, pharmacology, pharmaceutical chemistry, manufacturing and clinical studies that are assessed prior to allowing a medicine on the market to then monitor the safety of that medicine once it is in the market place. The evaluation process for a new medicine takes about 2 years of rigorous scientific analysis of hundreds of thousands of pages of documents. Even with that process, it is well understood that not all the side effects that could occur with a new medicine or vaccine will have been identified before it is released to the market. The effective monitoring of the medicine or vaccine requires knowledge of what sorts of side effects are likely to occur, effective adverse event reporting and analysis systems, and the legislative powers to take swift and appropriate action where a safety issue has been identified. All developed countries have invested these responsibilities in their medicines regulatory agencies. In the case of Australia these responsibilities lie with the TGA and are set out in the Therapeutic Goods Act 1989.
The Stokes Review incorrectly points to the situation that exists in the United States, where the US FDA has all the responsibilities that the TGA has for both approving and monitoring vaccines, but where there is also the Centers for Disease Control and Prevention (CDC) that have a role in assisting in designing the national vaccination program in the US, as an example of a different regulatory framework. The Stokes Review confuses the role of the CDC with that of the FDA and TGA and incorrectly implies that Australia has a medicines regulatory framework that is out of step with international norms, and impaired by a fundamental conflict of interest in its licensing and monitoring functions. This is not the case. Both the TGA and the US FDA have full responsibility for licensing and monitoring of all medicines and vaccines.
In the US, the Vaccine Adverse Event Reporting System (VAERS) provides a mechanism for the collection and analysis of adverse events associated with vaccines currently licensed in the US. It is run by a contractor, under the supervision of both the FDA and CDC, and both the FDA and CDC have access to VAERS data and use this information to monitor both the safety of individual vaccines (FDA) and the effectiveness of the vaccine program (CDC). However regulatory decision making remains the responsibility of the national regulatory authority, the FDA. There is a direct parallel between the situation in the US and in Australia, where the TGA is responsible for monitoring the safety of the individual vaccines, and the Office of Health Protection (also in the Department of Health and Ageing) which runs the National Immunisation Program is responsible for monitoring the effectiveness of the vaccine program. The VAERS database is analogous to the National Adverse Event database run by the TGA which receives reports from State and Territory vaccine programs, healthcare providers and consumers in order to enable the detection of safety signals with vaccines.
Based on an inaccurate characterisation of the role of the US FDA and the US CDC, the Stokes Review recommends the separation of licensing and monitoring functions for vaccines (and presumably by all medicines) in Australia. This would have significant deleterious effects on the ability of Australian health authorities to respond to emerging vaccine safety issues and is likely to delay, rather than expedite, the response to any issue. Presumably under the Stokes proposal, in order to understand the risks and benefits applying to the use of the vaccine on the market, any new vaccine monitoring authority would need to redo the initial scientific evaluations done by the licensing authority when they detected a safety signal so that they could understand the mechanisms causing the safety problem they detected. They would then need to negotiate with the licensing authority to amend any of the approved indications for which the vaccine was approved or to add any warning statements about the problem to Product Information or Consumer Medicine Information.
The separation of licensing and monitoring functions would require a significant amount of additional resources, the establishment of duplicated systems of evaluation and laboratory testing and the need for a doubling of the number of scientific experts involved in regulatory activities in this country. It would also take Australia well away from the well-established international norms for how medicines and vaccines are effectively regulated.
It is important to note that the World Health Organization (WHO) considers that "in all vaccine-producing countries and in all other countries where a national regulatory authority (NRA) exists, the NRA must be involved in immunization safety"1. This dual role is critical in being able to respond promptly to safety signals, reassess risk benefit balance, add warnings to Product Information (as in this case) or withdraw a product based on the review of adverse events.
1 World Health Organization, Immunization Safety Surveillance. Manila: Immunization Focus, Western Pacific Regional Office; 1999: http://whqlibdoc.who.int/wpro/1994-99/WPRO_EPI_99....
Had the Stokes Review properly understood the role of the CDC and the nature of the Australian vaccine framework, it would have become apparent that many of the vaccine program design and research roles that are played by the CDC in the US are undertaken by Commonwealth bodies such as the Australian Technical Advisory Group on Immunisation (ATAGI), the Australian Influenza Vaccine Committee (AIVC) and the National Centre for Immunisation Research and Surveillance. It would also have understood that there are no inherent conflicts of interest in the TGA evaluating vaccines to ensure they are safe enough to be on the market, establishing risk management protocols and monitoring the safety of the vaccines once they are on the market.
In addition, the TGA's post-market decision-making independence is addressed both through the legislative requirements contained within the Therapeutic Goods Act 1989 and through the structural and governance arrangements within the TGA.
Officers within the TGA carry out their regulatory responsibilities in accordance with the requirements of the Therapeutic Goods Act. The post-market safety surveillance functions of the TGA are clearly separated from the pre-market approval process in order to avoid any perceived conflict of interest between officers charged with initially assessing the suitability of a product and those charged with monitoring its ongoing suitability.
As well as casting doubts upon the world's medicines regulatory framework (by claiming that there is an inherent conflict of interest in licensing and monitoring functions), the Stokes Review states that the TGA "is essentially funded by the pharmaceutical companies and manufacturers of medical devices". This incorrectly implies that companies have some inappropriate commercial influence over the TGA.
In fact, the TGA is part of the Australian Government Department of Health and Ageing. The TGA requires commercial companies that apply for marketing approval to pay for the cost of the review of the application on a cost recovery basis. This process is common throughout government regulatory authorities and ensures that the companies that stand to benefit commercially from the work of regulatory authorities fund the full costs of pre-market evaluation and post-market safety monitoring rather than the Australian taxpayer. These cost-recovery arrangements are a matter of Australian Government policy not determined by the TGA.
Appropriate safeguards have been established to ensure that commercial considerations are not taken into account by the TGA in any of its regulatory decision-making. The Therapeutic Goods Act specifically prohibits influence by companies over the deliberations of the approval of medicines and there are rigorous safeguards in place that ensure that staff employed at the TGA, and expert advisory committee members involved in providing advice to the TGA, declare all real, perceived, pecuniary and non-pecuniary conflicts of interest that may be relevant in their advice. The TGA rigorously enforces these conflict of interest requirements. It is worth noting that, contrary to some of the public commentary that has occurred following the release of the Stokes Review, the TGA's conflict of interest requirements are more rigorous than other major regulatory authorities around the world and reflect Australia's commitment to sound public sector governance.
6. Reporting by the WA Department of Health and governance arrangements
The Stokes Review notes that "it is clear that the function and composition of the State Communicable Disease Control Directorate needs review to make it function less as a silo and disconnected hierarchy in the Department of Health and to share its information in a more timely fashion with the Department, health providers and the public."
The Review also notes that in regard to the manner of alerts passed on by CDCD, "word of mouth comments as notification are unacceptable and may lead to misinterpretation and risk."
Reporting and governance arrangements within the WA Department of Health are matters for the WA Government to consider and the TGA will not comment on the numerous references and recommendation within the Stokes Report regarding these.
Conclusion
Sadly, perhaps due to the short timeframe in which the Stokes Review was conducted and the lack of direct interaction with the investigating authorities, it contains significant errors, misinterpretations and flawed conclusions. Moreover, it fails to address the major issues arising from a program of childhood influenza vaccination that is unique in Australia.
The childhood influenza vaccine trial program implemented by the WA Department of Health was associated with a higher than expected rate of side effects in 2010 than in previous years. Despite a lack of specific monitoring processes within WA to effectively manage a program outside the National Immunisation Program (NIP), the relevant Commonwealth authorities responded in a timely and effective manner to prevent further harm and investigate the cause of the adverse events.
Writing in the journal Eurosurveillance, several of the senior WA public health officials responsible for the trial vaccine program noted that "the benefit-risk profile (of childhood influenza vaccination programs) would be improved if only children who were at increased risk of hospitalisation following influenza infection were targeted for vaccination"2. This conclusion points to the wisdom of the Australian Government's National Immunisation Program, which recommends the use of seasonal vaccine in this age group only in children at increased risk of influenza infection.
2 Kelly H, Carcione D, Dowse G, Effler P. Quantifying benefits and risks of vaccinating Australian children aged six months to four years with trivalent inactivated seasonal influenza vaccine in 2010. Euro Surveill. 2010;15(37):pii=19661.
It is an unfortunate fact that all medicines have the potential to cause side effects. In the case of the 2010 seasonal influenza vaccine, an increase in the expected rate of febrile convulsions led to Commonwealth and State authorities launching a rapid and far reaching investigation that prevented further harm once the problem had been identified. There are important lessons for vaccine programs arising from this investigation:
- There is a need to ensure prompt reporting of adverse events to the TGA to allow an effective and timely national response.
- There is a need for States to have well-considered monitoring programs in place well before embarking on any large scale trial vaccine programs outside the national framework.
- There is a need to ensure the public are provided with accurate, factual information to allow them to make informed decisions about the use of vaccines.
- There is a need for those charged with reviewing such events to ensure they have an accurate understanding of the situation before arriving at conclusions that are not supported by the facts and may do more harm than good.
- There needs to be greater public awareness of the mechanisms to report adverse events, and of the effective mechanisms in place in Australia to respond rapidly to emerging safety signals with any medicine.
The TGA will continue to work with consumers, healthcare professionals, and State and Territory health authorities to ensure that the lessons learned from the 2010 seasonal influenza vaccination campaign in WA are applied to prevent similar occurrences in future.

