Consumers and health professionals are advised that Australasian Medical and Scientific Ltd (AMSL), in consultation with the TGA, has initiated a recall for product correction for Contact Detach infusion sets manufactured before May 2014 (see below for a list of affected lot numbers).
These products are not being removed from the market. Consumers will have the option of having the affected devices replaced free of charge or continue using them in accordance with some additional instructions.
Contact Detach infusion sets are used in conjunction with a pump to deliver a continuous dose of insulin to help people with diabetes maintain normal blood glucose levels.
There has been a slight increase in reports of cases where the needles of these infusion sets have broken during use.
If this occurs, insulin delivery is interrupted, but the pump will not alarm to notify the user. The interruption of insulin delivery can cause hyperglycaemia, which if left untreated can cause serious health complications.
If a needle breaks during use it can also lead to infection.
In a small number of cases reported, a needle break has led to hospitalisation of the user to manage their blood glucose levels and/or to remove the needle.
The affected lot numbers are:
- 216296
- 218110
- 218111
- 219075
- 220163
- 224283
- 224284
- 224285
- 224286
- 226940
- 227063
- 656782
- 4263307
- 4263308
- 4263329
- 4263475
- 4263517
- 4263529
- 4263551
- 4263571
- 4263665
- 4263718
- 4263750
- 4263778
- 5000119
- 5001718
- 5003702
- 5006568
- 5006571
- 5007991
- 5009947
- 5013531
- 5013532
- 5015057
- 5018234
- 5020466
- 5020536
- 5021240
- 5022268
- 5025141
- 5026515
- 5029022
- 5029137
- 5031875
- 5031883
- 5031887
- 5031917
- 5031922
- 5031923
- 5031928
- 5031929
- 5031930
- 5034061
- 5034231
- 5034232
- 5036348
- 5038182
- 5038183
- 5038184
- 5038187
- 5038188
- 5040990
- 5040992
- 5045147
- 5045148
- 5047241
- 5047242
- 5047243
- 5047245
- 5054465
- 5054553
- 5054554
- 5057103
- 5057104
- 5057105
- 5058637
- 5058733
- 5058734
Information for consumers
AMSL has written to Contact Detach infusion set users to notify them of this issue and the actions they can take to address it.
If you or someone you care for uses Contact Detach infusion sets, check the box or pouch labels to see if you have an affected device. These labels include a reference number relating to the lot number.
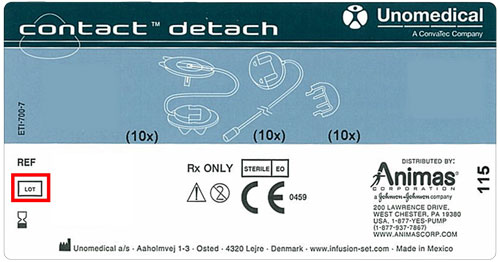
If the reference number matches one of the affected lot numbers listed above, you should take one of the two following alternatives:
Replacement - Call AMSL on 02 9882 3666 to arrange replacement of the infusion sets free of charge.
Continue use - You can continue to use the affected products if you carefully review the Instructions for Use included with the product, as well as follow the below additional instructions:
- Prior to use:
- Carefully remove the needle guard before inserting the infusion set. The needle guard should be removed without the use of any twisting or bending of the needle guard.
- Do not use the infusion set if the needle is bent or has been damaged.
- Do not bend the needle prior to insertion.
- Consult your health professional for the proper insertion site.
- During use:
- As always, it is essential to monitor your blood sugar levels frequently using your blood glucose meter.
- After use:
- Carefully remove the infusion set after use to avoid twisting or bending of the needle.
- Ensure the complete needle is present on the used infusion set before discarding it.
- Contact your health professional if you suspect that a needle has broken off and remained under the skin.
- Continue to monitor your blood sugar.
If you have any questions or concerns about this issue, speak to your diabetes nurse educator or other health professional. Alternatively, you can contact AMSL on 1300 851 056.
Information for health professionals
AMSL has written to relevant health professionals and consumers for whom it has contact details advising of this issue.
If you are treating patients who use Contact Detach infusion sets, ensure they are aware of this issue and the actions they can take to address it (see the 'Information for consumers' section above for specific instructions).
If you have any questions or concerns about this issue contact AMSL on 1300 851 056
Reporting problems
Consumers and health professionals are encouraged to report problems with medical devices. Your report will contribute to the TGA's monitoring of these products. For more information see the TGA Incident Reporting and Investigation Scheme (IRIS).
The TGA cannot give advice about an individual's medical condition. You are strongly encouraged to talk with a health professional if you are concerned about a possible adverse event associated with a medical device.

