Consumers and health professionals are advised that Nutricia Australia, in consultation with the TGA, is undertaking a recall for product correction for Flocare transition giving sets (male luer lock [MLL] and mobile giving sets only) with ENFit transition adaptors.
Flocare transition giving sets are a medical device used as part of an enteral tube feeding system, which delivers nutritionally complete feed (containing protein, carbohydrates, fat, water, minerals and vitamins) directly into the patient's stomach or small intestine.
They can be used in a patient's home or in a health facility setting.
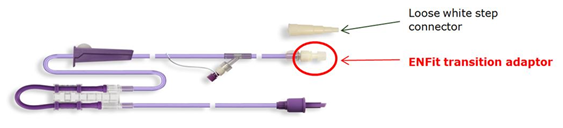
There have been some overseas reports of ENFit transition adaptors, which are supplied in Australia as a component of Flocare transition giving sets, leaking and/or breaking. As of 15 January 2016, there has been one report of this problem occurring in Australia.
This issue only affects a very small number of patients who use MLL and mobile giving sets and utilise the white transition adaptor on the patient-end to connect to a luer feeding tube. This configuration is not widely used in Australia.

ENFit transition adaptor
In most Australian situations, users connect the transition giving set to the feeding tube with a white step connector or directly to an ENFit feeding tube. In these situations the problem will not occur, as users are instructed to unscrew and discard the ENFit transition adaptor.
If a transition adaptor is used and it leaks or breaks, it could lead to patients receiving less feed than intended. For some patients, especially critical and/or volume sensitive patients, this can have a negative impact on their health if it not noticed for a significant period of time. Leakage can occur immediately after connection or over time.
To address this issue, Nutricia Australia is providing additional information advising users on how to minimise the risk of leakage and/or breakage.
Product improvements are also being undertaken, with updated products expected to be available in April 2016.
Information for consumers
If you or someone you care for uses Flocare transition giving sets for tube feeding, you can continue to use them. These products are not being removed from the market.
This issue only affects you if you use MLL and mobile giving sets that have a white transition adaptor on the patient-end which connects to a luer feeding tube. It does not apply to situations in which the transition adaptor is unscrewed and discarded during connection.
However, if this issue does affect you, you are advised to carefully check transition adaptors for any minor defects, avoid over-tightening connectors, and monitor for leakage after initial connection and again about two hours after connection. If any problems are identified or suspected, replace the transition giving set with a new one.
If you have any questions or concerns about this issue, speak to your health professional. Alternatively, you can contact Nutricia Australia on 1800 060 051.
Information for health professionals
If you are treating a patient who uses Flocare transition giving sets (MLL and mobile giving sets only), advise them of this issue including the specific circumstances in which leakage and/or breakage can occur.
Advise affected patients to carefully check transition adaptors for any minor defects, avoid over-tightening connectors, and monitor for leakage after initial connection and again about two hours after connection. If any problems are identified or suspected, they should replace the transition giving set with a new one.
If you have any questions or concerns about this issue, contact Nutricia Australia on 1800 060 051.
Reporting problems
Consumers and health professionals are encouraged to report problems with medical devices. Your report will contribute to the TGA's monitoring of these products. For more information see the TGA Incident Reporting and Investigation Scheme (IRIS).
The TGA cannot give advice about an individual's medical condition. You are strongly encouraged to talk with a health professional if you are concerned about a possible adverse event associated with a medical device.

