Consumers and health professionals are advised that Novo Nordisk, in consultation with the TGA, is recalling four batches of GlucaGen HypoKits. Patients who return affected products will be given free replacements.
GlucaGen HypoKits are used to treat severe hypoglycaemia (low blood sugar), in situations where the patient becomes unconscious or is unable to ingest a source of sugar.
It has been identified that a small number of GlucaGen HypoKits from four batches have needles which may become detached from the pre-filled syringes.
The affected batch numbers are:
- FS6X465
- FS6X536
- FS6X715
- FS6X891
The expiry date for all affected batches is 31 August 2017.
The batch numbers are printed on the GlucaGen HypoKit as indicated in the below photos.
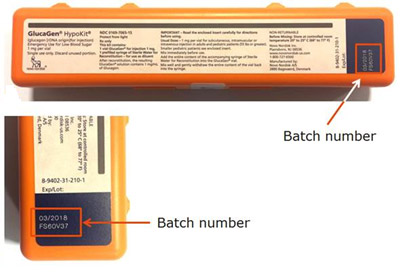
Because GlucaGen HypoKits are used during episodes of serve hypoglycaemia, it is important that patients have a functioning syringe. If the needle becomes detached from the syringe, the patient may not receive effective treatment and experience further serious health consequences.
Information for consumers
If you or someone you provide care for uses GlucaGen HypoKits, check any unused products to see if they are from one of the affected batches (see above for batch numbers and location of this information on the product label).
If you have a GlucaGen HypoKit from an affected batch return it to your pharmacy. You will be given a free replacement either immediately (if the pharmacy has unaffected stock available) or within a few days (if the pharmacy has to await resupply). If you do not receive a replacement immediately, retain your GlucaGen HypoKit until the replacement can be provided, as the likelihood of a detached needle is very low.
Please note, if you have a GlucaGen HypoKit with a batch number that is not specified above, it is not affected by this issue and you do not need to return it.
If you have any questions or concerns about this issue, speak to your diabetes nurse educator or other health professional. Alternatively, you can contact Novo Nordisk on 1800 668 626.
Information for health professionals
Novo Nordisk has written to health professionals to provide further information about this recall, and is providing a patient information leaflet that may be useful to help explain the issue.
If you have prescribed a GlucaGen HypoKit for a patient, contact them and ensure they are aware of this issue and advise them accordingly.
If you have any unused stock of GlucaGen HypoKits, check those products to see if they are from one of the affected batches (see above for batch numbers and location of this information on the product label). Quarantine all affected products and contact your supplier to arrange their return.
If you have any questions or concerns about this issue, contact Novo Nordisk on 1800 668 626.
Reporting problems
Consumers and health professionals are encouraged to report problems with medicines or vaccines. Your report will contribute to the TGA's monitoring of these products.
The TGA cannot give advice about an individual's medical condition. You are strongly encouraged to talk with a health professional if you are concerned about a possible adverse event associated with a medicine or vaccine.

