Consumers and health professionals are advised that the TGA is urgently working with sponsor Physio-Control to address an issue involving Infant/Child Reduced Energy Defibrillation Electrodes.
These defibrillation electrodes are used with the following automatic external defibrillators (AEDs) and are for use on infants or children:
- Lifepak Express AED
- Lifepak CR Plus AED
- Lifepak 1000 Defibrillator
- Lifepak 500 Biphasic AED that have a pink connector.
AEDs are portable electronic devices that automatically diagnose and treat cardiac arrhythmia (also known as abnormal or irregular heartbeat) which can lead to cardiac arrest.
Defibrillation is the application of electrical therapy to stop the arrhythmia, allowing the patient’s heart to re-establish an effective rhythm.
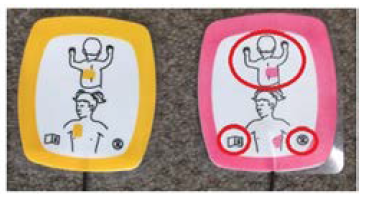
It has been identified that artwork on Infant/Child Reduced Energy Defibrillation Electrodes show incorrect electrode placement for an infant.
The incorrect artwork (showing placement of the pink electrode on the infant’s back) is located on the defibrillation electrodes themselves, with the artwork on the external packaging being correct (showing placement of the pink electrode on the infant’s chest).
If the electrodes are placed incorrectly it could result in ineffective energy delivery to the patient and lead to serious injury or death.
There are no issues with the performance or function of the defibrillation electrodes.
Correct artwork

Incorrect artwork
The TGA is aware that 75 units of these defibrillation electrodes have been distributed in Australia.
Information for facilities
The TGA is working with Physio-Control to address this issue, and a recall will be initiated in the next few days. Further information will be published when it becomes available.
In the meantime, facilities (including hospitals and potentially ambulance services) that have one or more of the Lifepak AEDs listed above should check the defibrillation electrodes on hand to verify if they have the incorrect placement diagrams.
Users should be aware of the correct electrode placement instructions as shown above and on the external packaging.
The pink electrode must be placed on the infant’s bare chest (not on their back).
Reporting problems
Consumers and health professionals are encouraged to report problems with medicines or vaccines. Your report will contribute to the TGA's monitoring of these products.
The TGA cannot give advice about an individual's medical condition. You are strongly encouraged to talk with a health professional if you are concerned about a possible adverse event associated with a medicine or vaccine.

