This Product recall is part of the Philips recall action for CPAP, Bi-Level PAP devices and mechanical ventilators collection. Visit the collection to see further progress updates.
3 January 2024
The recall action for the sleep apnoea and respiratory care machines is nearing a close with the majority of devices having now been replaced or refunded.
Philips continues to provide updates to us on the testing they have done on the sound-abatement foam in the devices and routinely update their website. The latest update
from Philips on the tests carried out on these devices can be found at the news on the Philips website.
It is noted in the latest information supplied by Philips that a correction has been made to the list of volatile organic compounds (VOC) potentially affecting users of the devices. The original list included dimethyl diazene but this is incorrect and the compound has now been confirmed as acetone.
This correction does not change the regulatory action taken in Australia.
A reminder - if you use a Philips Continuous Positive Airway Pressure (CPAP) or a Bi-level Positive Airway Pressure (Bi-Level PAP) machine and you have not yet registered with Philips to obtain a replacement device or refund, register as soon as possible on the Philips website.
8 July 2022
Mid-year progress update
Over 60% of the affected devices registered with Philips in Australia have now been corrected under the repair/replacement program.
There have been some delays to devices being corrected. This is due to a variety of reasons, some of which are beyond Philips' control. We are working with Philips to minimise these delays where possible.
Despite the delays, Australian patients are receiving their corrected devices ahead of many global markets.
BiPAP settings (patient script) issues
Some patients have received replacement BiPAP devices with settings (patient scripts) that have been incorrectly installed by Philips.
If you are experiencing any discomfort or your therapy does not seem to be effective, you need to have your therapy settings checked by either:
- Contacting Philips on 1800 009 579 or
- Contacting your sleep clinician.
Your BiPAP device has two types of settings:
- "Therapy setting" (patient script) which you cannot change. This must be done by Philips or your sleep clinician and
- "Comfort setting" which you are able to adjust to suit your individual needs by using the "My Settings" menu on your device.
Some patients who have received replacement BiPAP devices with incorrect script settings have been contacted by Philips and their devices have been fixed. Philips will be writing to all patients and working to ensure that all BiPAP device settings have been correctly installed.
If you are feeling unwell, seek medical attention.
Consumers and health professionals are encouraged to report problems with medical devices. Your report will contribute to the TGA's monitoring of these products. For more information see the TGA Incident Reporting and Investigation Scheme (IRIS).
Updates on repair/replacement program
Repair / replacement for all affected devices has commenced, except for:
- A Series BiPAP
- Omnilab and
- DreamStation Go devices.
For a full list of impacted devices refer to the table below (25 June 2021 update).
The TGA has taken regulatory action and fined Philips for the delay in providing important information. Philips are now required to meet weekly with the TGA to ensure that progress with the remediation plan remains on track and any further delays to the repair/replacement program are identified as soon as possible.
Philips recall action for CPAP, Bi-Level PAP devices and mechanical ventilators
Device repair/replacement program - progress update
16 December 2021
Approximately 20% of the affected devices registered with Philips in Australia for correction have now been corrected under the repair/replacement program. Users are reminded that registration at https://www.philipssrcupdate.expertinquiry.com is vital to ensure you receive the repair or replacement of your device.
In addition to the DreamStation CPAP and 50 Series CPAP, the repair/replacement program for DreamStation BiPAP devices has now also commenced. Philips has advised the repair/replacement for the following devices will also commence in December 2021-January 2022:
- DreamStation ASV
- DreamStation ST, AVAPS
- 50 Series ASV
- C Series ASV (60 Series)
- C Series S/T AVAPS (60 Series)
If you require assistance to set up your replacement device contact Philips at 1800 830 517 (Option 1). If required, Philips may arrange an in-clinic assessment to assist you.
You can verify that your device no longer has the impacted PE-PUR foam by checking the label for a 'UDI' field which is being included on all repaired devices (as shown in the before / after label pictures below):

Example of Trilogy label in use prior to rework for foam correction.
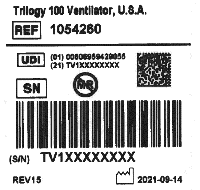
Example of Trilogy label template that will be affixed after rework for foam correction (UDI information present).
The label is found on the bottom of your device (or on the back of the Trilogy 100 devices).
In the event you have an upcoming surgical procedure and have not received a repaired or replaced device, advise your clinician prior to your procedure (as early as possible) that you use an affected device and speak with them about ongoing use following surgery.
Updates on the supply of CPAP, Bi-Level PAP devices and mechanical ventilators
Philips are currently working to manufacture enough devices for users affected by the issues with PE-PUR foam. Given this, they are unable to fulfil orders at this time for new patients requiring CPAP or Bi-Level PAP devices.
Supply of Trilogy Evo ventilators has resumed for new patients requiring ventilation, noting this model supersedes the Trilogy 100 model.
Philips recall action for CPAP, Bi-Level PAP devices and mechanical ventilators
Device repair/replacement program has commenced
18 November 2021
The repair/replacement program for the following Philips' devices commenced on 3 November 2021:
- DreamStation CPAP
- 50 series CPAP
Registered users are being contacted by Philips to confirm their details in the lead up to each device being corrected.
If you have received your replacement device and need assistance to set it up, visit the Philips website for support.
We will provide updates as the program progresses to include other models.
If your contact details have changed since you registered your device, notify Philips on 1800 009 579.
US-FDA inspection of Philips Respironics Inc
The US-FDA has recently published an update to their report on inspections of a Philips Respironics Inc manufacturing facility. The report outlines concerns with the risk identification process used to identify models subject to recall actions. There was also a question raised about the silicone-based sound abatement foam being used as a replacement for the PE-PUR foam.
Until further findings become available, the US-FDA have not advised of any change to the replacement program in the USA and Philips are continuing their repair and replacement program in Australia. Patients who have already had the PE-PUR foam replaced should continue use of their device, unless instructed otherwise by their doctor.
The TGA is working closely with international regulatory agencies, including the US-FDA, to ensure a consistent global approach to the identified safety concerns.
Recommendations for users with devices that have not yet been corrected remain unchanged.
We will provide further updates on this matter as new information becomes available.
Philips recall action for CPAP, Bi-Level PAP devices and mechanical ventilators
Consumer and regulatory updates
23 September 2021
The TGA has heard from many people affected by this action. We appreciate your feedback and recognise the seriousness of your concerns. Hearing from you assists us in our discussions with Philips to ensure the effectiveness of their proposed actions.
How long will it take for my device to be repaired? Can TGA speed this up?
The estimated start date for remediation activities in Australia was September; Philips has now stated that an initial delivery of DreamStation devices will be sent in late September and replacement work for these devices will start in mid-October. Philips' parent company has announced that the repair/replacement program is expected to be completed within approximately 12 months.
This recall is being coordinated globally and relies heavily on manufacturing capacity and importation of materials from overseas. The TGA will monitor the timelines outlined by Philips in their remediation plan and continue to raise issues that need to be addressed by the company.
We are also working with manufacturers of alternative devices to encourage increased supplies to be made available in Australia, noting the competition with other global markets for such devices.
What is the TGA's role in a recall action and what has TGA done for this issue?
A recall action should not commence in Australia until the proposed action and communication are agreed to by the TGA. We give this agreement when we are satisfied that the action effectively mitigates the risks posed by an issue with a therapeutic good.
As part of our assessment, we often seek expert medical or scientific advice. Shortly after this recall action was announced, we convened our Ventilator Expert Working Group, comprising state and territory health officials, external independent clinicians, biomedical engineers and peak health professional bodies such as the Australasian Sleep Association. The Working Group provides feedback on the appropriateness of the actions and communications from both the TGA and Philips.
After a recall commences, we monitor its effectiveness to ensure all affected product is fixed or removed from the market where this is needed. In some instances, further investigations or enforcement actions may also occur.
Why is this a 'product defect correction' rather than a 'recall'?
In reviewing Philips' proposed actions, we have concluded that in many instances total removal (recall) of the affected devices is not possible unless sufficient replacements are available. We are aware there are limited supplies of alternative devices and understand the risks posed by interrupting patient treatment. Recognising that individual needs differ, it is important that each patient seeks advice from their own healthcare professional.
We recognise and understand this places significant pressure on health professionals. The course of action for each patient, i.e. immediate replacement, adjustment, interruption, or continued use until repair/exchange, must be assessed in each case. The decision should be based on the currently available information on risks and individual patient circumstances (type and severity of respiratory disorder, age, comorbidities, symptoms, occupation, duration of therapy, etc.)
A 'product defect correction' may be called a 'recall' in some international markets and identified in this way on the websites of other regulatory agencies. However, the advice for users to contact a health professional before making changes to treatment, and the approach of repair / replacement of the devices, remains consistent across the world. We are working closely with international regulators on this Philips recall action to support a consistent approach.
Are other devices impacted by this issue?
The TGA has commenced a post-market review of other products in the 'sleep device' category included in the Australian Register of Therapeutic Goods (ARTG). This review will confirm if other devices are also affected by degrading sound-insulating foam. Information on the outcomes of this review will be published on our website.
Can I use an inline filter with my device?
At present, use of an inline bacterial filter has been recommended for mechanical ventilators only. The filter will provide a barrier to prevent users from inhaling the degraded foam particles, but it will not protect against the gases that may be released by the foam. If the filter becomes wet or clogged with debris it may reduce device performance and should be replaced frequently as referenced in the device's user manual.
Use of an inline bacterial filter is not currently recommended for CPAP / Bi-Level PAP devices.
Philips recall action for CPAP, Bi-Level PAP devices and mechanical ventilators
Consumer and regulatory updates
18 August 2021
The TGA continues to receive enquiries from consumers concerned about progress with this recall action. See the following information we have provided on common questions.
How are users being notified of the sound abatement foam issues?
Where possible Philips Electronics Australia Pty Ltd has sent letters directly to patients. In some cases, patients are not directly managed by Philips because other companies have taken on the responsibility of supplying the devices to customers. These distributor companies will be responsible for sending the letters to their customers. Who sends you the letter will be based on the information you have provided Philips or the place where you purchased your device.
In addition to direct patient letters, Philips have used a variety of ways to let people know about the recall. These include publication of information on the Philips' website, advertising the recall in print and digital newspapers and notifying relevant healthcare associations and patient support organisations.
The TGA has also notified key stakeholders including state and territory health departments, the Australian Government Primary Health Networks, consumer groups and associations. We have also sent information using our social media channels.
Various television and media outlets have published articles about this recall and users are sharing information across social media support groups. We remind all patients and their caregivers to use the TGA website for accurate information.
Does the replacement process require regulatory approval in Australia?
Some consumers are concerned that the Philips website includes advice that -
"the repair process for existing devices requires regulatory approval in your country, which we are working toward obtaining as quickly as possible. We will begin the repair/replacement process immediately upon that approval."
This statement only applies in some countries. The repair / replacement process does not require regulatory approval in Australia.
What is the current timeframe for my devices to be fixed or replaced?
Philips Australia is waiting for stock of replacement foam to arrive from overseas. This is expected to arrive in September for the DreamStation platform and the repair/replacement plan will start as soon as possible after this happens. The TGA will provide another update when corrective actions have commenced.
I have already purchased a new device, what should I do?
If customers have already purchased a replacement CPAP machine, register your affected device at www.philips.com/src-update and click on "Begin registration process". Philips will provide instructions on how to return your affected device.
I would like a refund or reimbursement
Philips is responding to consumer requests for refunds. If you are not satisfied with the refund or reimbursement offered, you should request the offer be reviewed. You can do this via the Philips Electronics Australia Ltd support hotline on 1800 009 579.
In addition, you have rights under the Australian Consumer Law which are entirely separate to any remedy Philips may provide as part of this recall action. You can find more information on the Australian Competition & Consumer Commission (ACCC) website about your consumer guarantee rights.
The TGA does not administer consumer law. You can make a consumer complaint to the ACCC if you experience difficulties asserting your rights regarding the Philips products covered by this recall action.
Philips recall action for CPAP, Bi-Level PAP devices and mechanical ventilators
Update - further information for consumers
16 July 2021
If you have not already done so, register your device at www.philips.com/src-update. This information is needed so the company can plan the repair/replacement program efficiently.
Some consumers have reported that they are not sure if their registration has been received. When you register you will get a registration number. If you don't receive this number, re-register your device. To help reduce this confusion, Philips is now sending a confirmation email with each new registration.
Other consumers have purchased their device overseas and are not sure if they should register here. If you currently live in Australia, regardless of the place of purchase, go to the Philips registration website and select 'Australia' before entering your serial number.
Towards the end of this month, Philips will begin contacting everyone who has registered their device. They will confirm your details and outline the type of correction proposed for your device. This may take some time given the large number of affected people. Depending on the device type and its age, Philips will either provide a new base unit device or replace the foam component.
We will continue to update you as further information becomes available.
Philips recall action for CPAP, Bi-Level PAP devices and mechanical ventilators
Update - Product defect correction
9 July 2021
The TGA is aware that some consumers are having difficulty contacting Philips about the safety concerns with their devices. The company has advised us that additional resources are being put in place to help with the high level of calls.
It is important that customers register their devices on the Philips website. This will help us plan an effective approach to either repair or replace them, taking into account the specific needs of various patient groups. Some additional guidance on registering is outlined below.
We will publish further information on repair and replacement timeframes as soon as this is available. The TGA is assessing safety information to ensure any proposed actions are appropriate - these may be different for different devices. Philips has confirmed that replacing the device or replacing the foam will be done at no cost to the patient.
It is strongly recommended that you do not attempt to remove the foam yourself and then continue using your device. Discuss your concerns with your health professional and register your device on the Philips website.
Information for consumers
You need to register your device on the Philips support website.
Follow these steps:
Go to www.philips.com/src-update and click on "Begin registration process"
(For further information, you can also scroll down to the "Patient, Users, or Caregivers" heading and then click on "Begin registration process")
- Select your user or customer category and choose "Australia" as the country (even if you purchased your device overseas)
- Continue to follow the remaining steps, ensuring you enter your device's Serial Number to check if your device is affected
- If your device is affected, you will be asked to register your device.
Experiencing problems
If you are experiencing any of the symptoms listed below, talk with your health professional. We also encourage consumers and health professionals to report problems with medical devices to the TGA.
We will publish further updates as new information becomes available, to continue providing support for all users, health professionals and customers impacted by this issue.
If you have any questions or concerns, contact Philips Electronics Australia Ltd on 1800 009 579. Further information is available at www.philips.com/src-update.
Philips recall action for CPAP, Bi-Level PAP devices and mechanical ventilators
Product defect correction - safety hazard caused by foam degradation and emissions
6 July 2021
Philips Electronics Australia Ltd, following consultation with the TGA, is undertaking a product defect correction for the 14 sleep and respiratory care devices identified in the tables below. This is in response to risks posed by the polyester-based polyurethane (PE-PUR) sound abatement foam component in these goods.
Philips has identified that the foam used in the blower boxes of their continuous and non-continuous ventilators may degrade into particles, which may enter the devices' air pathway and be ingested or inhaled by the user, and release certain chemicals as gases. The foam may degrade more quickly if the device is cleaned using unapproved cleaning methods, such as ozone gas products.
Where possible, Philips will replace the sound abatement foam in affected devices. If this is not possible, a replacement device will be provided.
Information for consumers
Check the list of devices lower on this page to see if your device is affected by this action.
Do not stop using your device without speaking to your physician or care provider. Stopping treatment suddenly could have an immediate and detrimental effect on your health.
You need to register your device on the Philips support website: www.philips.com/src-update. This will allow Philips to contact you to organise the corrective actions or a replacement device.
If you have any questions or concerns, contact Philips Electronics Australia Ltd on 1800 009 579. Further information is available at www.philips.com/src-update.
If your device is an affected CPAP or bi-Level PAP unit
Make an appointment with your physician or care provider before making any changes to your prescribed therapy. Together with your physician, determine if the benefit of continuing therapy with your device outweighs the risks identified and discuss alternative long-term therapy options.
Make sure you follow the recommended cleaning and replacement guidelines in the operating manual for your CPAP machine and accessories.
If your device is an affected mechanical ventilator
Do not stop or alter your prescribed ventilator therapy.
Make an appointment with your physician or care provider before making any changes to your prescribed therapy. Together with your physician, determine if the benefit of continuing therapy with your device outweighs the risks identified and discuss alternative long-term therapy options.
You can use an inline bacterial filter to help reduce the risk of inhaling particles of foam. These filters will not reduce exposure to the gases that may be released by the degrading foam.
Guidance on installation of the filter is found in the device's operating manual. It is important to install these correctly as a filter may increase the resistance of air flow.
Information for health professionals
Be aware of the safety concerns associated with these devices and advise patients accordingly. A patient-specific risk assessment may be needed to determine whether the benefit of continuing therapy outweighs the risks identified.
If you have any questions or concerns about this issue, contact Philips Electronics Australia Ltd support hotline on 1800 009 579. Further information is available at www.philips.com/src-update.
Risk assessment outcomes
Philips' investigations identified that the majority of particles are of a size unable to penetrate into deep lung tissue and are likely to remain in the patient's upper airway. Only particles with a diameter of <1-3 μm may penetrate into the lower respiratory tract.
Analysis of the degraded foam demonstrates the particles may include compounds such as diethylene glycol (DEG), toluene diamine isomers (TDA) and toluene diisocyanate isomers (TDI). Philips is undertaking investigations to assess whether the amount of degraded PE-PUR foam inhaled and/or ingested by a patient may potentially exceed the tolerable intake for these compounds.
The foam may also emit certain chemicals (volatile organic compounds - VOCs). Product testing has shown that this 'off-gassing' mostly occurs during initial operation, but may possibly continue throughout the device's useful life.
The potential risks associated with these issues include: headache/dizziness, irritation (eyes, nose, respiratory tract, skin), inflammatory responses, asthma, hypersensitivity, nausea / vomiting, adverse effects to other organs (e.g. kidneys and liver) and possible carcinogenic effects.
The complaint rate associated with these issues was 0.03% for the year 2020. To date, there is no definitive evidence of long-term harm to patients and there have been no reports of death.
Update - Philips recall action for CPAP, Bi-Level PAP devices and mechanical ventilators
Safety hazard caused by foam degradation and emissions
25 June 2021
The TGA is now able to confirm that the following devices are affected by this action.
All devices manufactured before 26 April 2021 All serial numbers | |
|---|---|
| Continuous Ventilator, Minimum Ventilatory Support, Facility Use | E30 (Emergency Use Authorization) |
| Continuous Ventilator, Non-life supporting | DreamStation ASV |
| DreamStation ST, AVAPS | |
| SystemOne ASV4 | |
| C-Series ASV | |
| C-Series S/T and AVAPS | |
| OmniLab Advanced+ | |
| Noncontinuous Ventilator | SystemOne (Q-Series) |
| DreamStation | |
| DreamStation Go | |
| Dorma 400 | |
| Dorma 500 | |
| REMstar SE Auto | |
All devices manufactured before 26 April 2021 All serial numbers | |
|---|---|
| Continuous Ventilator | Trilogy 100 |
| Trilogy 200 | |
| Continuous Ventilator, Minimum Ventilatory Support, Facility Use | A-Series BiPAP V30 Auto |
| Continuous Ventilator, Non-life supporting | A-Series BiPAP A40 |
| A-Series BiPAP A30 | |
If your device is an affected mechanical ventilator, use of an inline filter will reduce the risk of inhaling particles of foam. Guidance on installation of the filter is found in the device's Instructions for Use document (or 'operating manual').
We re-iterate our earlier advice that, in critical or lifesaving situations, patients or caregivers should continue using the devices until an alternative becomes available. Patients should speak with their health professional prior to making any changes to their prescribed therapy.
Where possible, affected devices will have the sound abatement foam component replaced. Information on the timing of this correction is still being confirmed with Philips.
We will publish further information as soon as it becomes available, including advice that the recall action has commenced in Australia and the outcomes of TGA's risk assessment.
Philips recall action for CPAP, Bi-Level PAP devices and mechanical ventilators
Safety hazard caused by foam degradation and emissions
18 June 2021
The TGA is working with Philips on its global recall action for Continuous Positive Airway Pressure (CPAP), Bi-Level Positive Airway Pressure (Bi-Level PAP) devices and mechanical ventilators due to risks posed by the polyester-based polyurethane (PE-PUR) sound abatement foam component in these devices.
The majority of the affected devices are in the first-generation DreamStation product family.
A full risk assessment is underway including numbers supplied in Australia and on potential particulate and chemical exposure risks which will inform further advice to health professionals and consumers.
As a precautionary measure, pharmacies are advised to quarantine and remove from sale any unsold units of the affected devices.
We will continue to work with Phillips to ensure that comprehensive information is made available as quickly as possible through formal published announcements on the TGA website and from Phillips directly to customers.
Information for consumers
For devices currently in use in critical or lifesaving situations, patients or caregivers should continue using the devices until an alternative becomes available.
Information for health professionals
Be aware of the above issue and advise patients accordingly if they seek advice.
Reporting problems
Consumers and health professionals are encouraged to report problems with medical devices. Your report will contribute to the TGA's monitoring of these products. For more information see the TGA Incident Reporting and Investigation Scheme (IRIS).
The TGA cannot give advice about an individual's medical condition. You are strongly encouraged to talk with a health professional if you are concerned about a possible adverse event associated with a medical device.

