PIP breast implants - background
PIP breast implants are medical devices manufactured by a French company, Poly Implant Prothèse (PIP).
PIP breast implants are known to have been used in Australia from September 1999 until April 2010, when non-implanted PIP breast implants were recalled from the market following advice from the French regulator (AFSSAPS) that in making these devices the manufacturer had used unapproved materials which may affect their safety and performance.
The Chief Medical Officer, Professor Chris Baggoley, has published a report and two fact sheets about PIP breast implants. This information is available at:
- Poly Implant Prothèse (PIP) Breast Implants: Report of the Chief Medical Officer
- PIP Breast Implants and Breast Feeding Advice from the Chief Medical Officer - July 2012
- Fact Sheet - Silicone gel filled Breast Implants.
On 1 February the UK regulator, the Medicines and Healthcare Products Regulatory Authority (MHRA), published its final report on PIP silicone breast implants. Their report is available at MHRA toxicology testing and collection of clinical findings upon removal of the implant.
The report details the analytical and toxicological work undertaken by MHRA and provides an analysis of the reports received in relation to ruptures and clinical observations from explanted PIP breast implants. The TGA has reviewed the report and determined that there is no new information that requires a change to the current advice being provided to Australian women with these implants.
Testing undertaken by TGA has not found evidence that the risks involved with the use of PIP breast implants are any greater than those for any other brand of silicone gel-filled breast implants.
Information for consumers
The Australian Government's advice remains that removal of PIP breast implants in the absence of evidence of rupture is not routinely required.
It is important that decisions made by patients and their treating doctors about the need for further surgery are fully informed by the best available evidence, and take each individual patient's circumstances fully into account.
People with PIP implants, and those who are unsure about the brand of their breast implants, are strongly encouraged to consult their general practitioner (GP) or surgeon for individual clinical assessment and advice.
TGA regulates therapeutic goods and cannot give individual clinical advice. If you suspect your symptoms are associated with your implant you should also report this to the TGA, or ask your health care professional to report it for you.
Information for consumers and patients with breast implants, including a series of videos, is available from HealthDirect.
Medicare rebates
The Medicare rebate for MRI services for patients with PIP breast implants has been extended to 12 March 2015.
Normal Medicare arrangements apply to reimbursing patients for the usual cost of medical services related to dealing with health concerns about their PIP implants, including ultrasound examinations and any consultations with their GP or surgeon.
Women who know that they have PIP breast implants, or where clinical advice is that they might have PIP implants, are now able to access Medicare rebates for MRI services examinations to accurately assess the state of their implants.
Medicare rebates are also available for the removal and replacement of breast implants where the treating clinician (surgeon) believes there are physical (e.g. rupture) and/or psychological (e.g. significant anxiety) indications for such surgery. Medicare rebates contribute to the fees charged by doctors, including those of the surgeon, anaesthetist and any surgical assistants, but Medicare rebates do not cover the cost of replacement breast implants. Patients with private health insurance should contact their insurer to ascertain if their policy would cover the cost of the implant.
Medicare also does not cover private hospital accommodation and hospital theatre costs, which may also be subsidised by private health insurance.
Patients may elect to be referred by their medical practitioner to the nearest appropriate public hospital. The hospital specialist can then advise the patient about the best course of action which may include surgical treatment.
Update on TGA testing of PIP breast implants
The TGA has performed and reported on a large number of tests on the chemical properties, physical properties and the biological safety of the gels and shells of PIP breast implants.
Please refer to the Update on TGA testing of PIP breast implants, 11 February 2013 for detailed information on:
- tests on new implants (conducted since the last report on 2 April 2012)
- tests on explanted PIP implants.
Reports of problems with PIP breast implants
Approximately 13,000 PIP silicone gel breast implants were supplied in Australia between 1998 and 2010.
The table below shows the number of instances of ruptured PIP breast implants notified to the TGA as of 31 January 2013.
| Reporter | Confirmedi ruptures | Unconfirmedii ruptures |
|---|---|---|
| surgeons | 320 | 10 |
| patients | 107 | 12 |
| supplier | 24 | |
| All reporters | 451 | 22 |
- The TGA categorises ruptures as 'confirmed' if there is sufficient information to uniquely identify:
- the patient
- the implant used
- that an X-Ray or other diagnostic image showed that the device was ruptured; or the implant was found to be ruptured when it was removed.
- The TGA has sought further information but as yet has not received sufficient information to uniquely identify the rupture.
The figures for confirmed and unconfirmed implant ruptures are updated as the TGA receives additional information. As a result of this further, more detailed information, the confirmed/unconfirmed rupture figures may increase or may decrease.
The unconfirmed ruptured implant figure continues to reflect those reports received by the TGA where insufficient information has been provided to confirm the presence or absence of a rupture. In this situation the TGA requests additional information to clarify whether an implant has ruptured; this information would be imaging results (MRI or ultrasound) and/or a description of the implant when it is surgically removed.
The reports of PIP implant rupture received by the TGA to date suggest that the average time to rupture is between 5 and 6 years, (see Figure 1) even though there is a small cluster of implants that were implanted for ten years before they ruptured.
Figure 1: Time to rupture for PIP implants reported to the TGA where a date of implantation and a date of removal was provided.

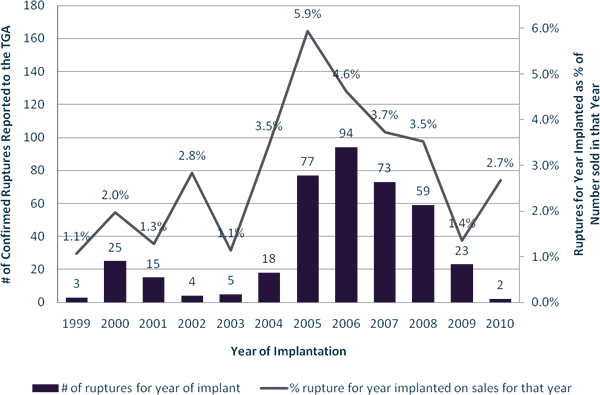
Figure 2: Number of ruptures (at any time after implantation) by year of implantation and % rupture rate by year of implantation as a fraction of implants sold in that year.
Limitations of the data displayed in Figures 1 and 2
The TGA only holds data for reports it has received, the majority of which came in 2012 following heightened publicity and requests by TGA for surgeons to make reports about PIP implants. TGA does not hold comprehensive rupture data for all PIP silicone breast implants including data on ruptured implants that may have occurred prior to the stimulated reporting that occurred in 2012.
Also, the data is rounded to whole years and therefore lacks some precision. For example, Figure 2 shows ruptures for a given year as a percentage of implants sold by the supplier in that year. Furthermore, being sold in a particular year does not necessarily equate to the product being implanted in that year but it is a reasonable assumption.
Reporting problems with breast implants
Patients and healthcare professionals are strongly encouraged to report problems with medical devices including breast implants to the TGA.
In reporting adverse events associated with your breast implants, contact with your treating doctor is important to ensure that the TGA receives all the information that is required to officially confirm reported ruptures (or other problems) with these devices and to conduct further enquiries where necessary.

