On this page: Summary | Medicine shortage notifications | Publication of notifications | Section 19A approvals | Enhancements to the medicine shortages scheme
Summary
The Therapeutic Goods Act 1989 was amended effective 1 January 2019 to introduce a mandatory reporting scheme for medicine shortages and permanent discontinuations of all prescription medicines and certain over-the-counter (OTC) medicines on the Australian Register of Therapeutic Goods (ARTG). The scheme, administered by the Therapeutic Goods Administration (TGA) within the Department of Health, requires sponsors to report shortages to improve awareness and enable early action to minimise their impact.
The TGA actively liaises with sponsors of affected medicines and publishes information about all current and anticipated medicine shortages of critical impact on the Medicine Shortages Information Initiative (MSII) website. Information about low- and medium-impact medicine shortages is voluntarily published with the sponsors' consent, and information on the overwhelming majority of such shortages is published.
Since the introduction of mandatory reporting, the TGA has received significantly more notifications of medicines shortages than in previous years and has granted a larger number of approvals for supply of overseas-registered alternative products to mitigate the effects of shortages.
This report describes the first 12 months of the mandatory reporting scheme. Some statistical comparisons are made to 2018, the final year of the voluntary reporting scheme.
Key findings
- There was a 290% increase in the total number of shortage notifications reported to the TGA during 2019 compared with 2018.
- Sponsors consented to the publication of 92% of shortages notified to the TGA (75% consented in their initial notification with the remainder consenting following discussion with the TGA). The TGA published all critical shortage notifications.
- The number of approvals granted by the TGA under section 19A of the Act (for short-term supply of a product registered in a comparable overseas country to address a particular shortage) increased by 40% during 2019 compared with 2018.
- Engagement with stakeholders through the Medicine Shortages Working Party has identified the potential to further develop the scheme to:
- improve transparency by increasing the proportion of shortage notifications that are published by the TGA
- increase health professionals' awareness and use of the MSII website through communications activities and website enhancements
- better forecast shortages using more detailed information from sponsors and wholesalers
- more rapidly mitigate some shortages by improving information for sponsors about section 19A requirements.
Medicine shortage notifications
A medicine is in shortage when its supply in Australia will not, or will not likely, meet the demand for it at any time in the next six months for all the patients in Australia who take it or who may need to take it.
Sponsors must actively monitor the supply of their medicines and report any anticipated shortages within the legislated reporting timeframes using the electronic notification form provided by the TGA.
The electronic notification form collects details of the shortage, including information to assist the TGA to assess the patient impact of the shortage, the reasons for the shortage, the potential effects on supply of other medicines and the sponsor's proposed approach to managing the shortage.
In this section: Notifications of new shortages | Total notifications | Patient impact of new shortages | Shortage status
Notifications of new shortages
The number of new shortages notified to the TGA has increased from an average of 50 per month during 2018 to an average of 150 per month during 2019 (Figure 1).
From 1 January to 31 December 2019 there were 1797 new shortages reported, compared with 572 shortages reported in 2018. Note that there was also an anticipatory increase in voluntary reporting of shortages during the last 4 months of 2018. Shortages reported in 2019 were associated with 1415 different products. There were 382 products subject to repeated periods of shortage between periods of supply.

Figure 1 in table format
| Month | 2018 | 2019 |
|---|---|---|
| January | 26 | 282 |
| February | 30 | 190 |
| March | 28 | 165 |
| April | 25 | 92 |
| May | 41 | 206 |
| June | 11 | 97 |
| July | 28 | 131 |
| August | 25 | 89 |
| September | 47 | 129 |
| October | 58 | 149 |
| November | 69 | 117 |
| December | 184 | 150 |
Total notifications
Total notifications include notifications of new shortages and updates to previously notified shortages. Sponsors are able to update their notifications whenever information changes.
In 2019 the total number of notifications submitted was 5862 compared to 1493 in 2018. This was an increase from an average of 120 per month during 2018 to an average of 490 per month during 2019 (Figure 2).

Figure 2 in table format
| Month | 2018 | 2019 |
|---|---|---|
| January | 93 | 546 |
| February | 80 | 560 |
| March | 99 | 537 |
| April | 77 | 404 |
| May | 113 | 610 |
| June | 91 | 400 |
| July | 85 | 454 |
| August | 88 | 428 |
| September | 197 | 487 |
| October | 128 | 446 |
| November | 168 | 481 |
| December | 274 | 509 |
Patient impact of new shortages
The Medicines Watch List is a legislative instrument used to simplify and streamline decision-making when determining the impact of a shortage. Any shortage of a medicine listed on the Medicines Watch List is automatically considered to have a critical patient impact.
Shortages of other medicines are considered to have a critical patient impact if:
- at the time of the shortage there are no products on the ARTG that could be used as a substitute, or if an appropriate substitute product is unlikely to be available in sufficient quantities to meet demand, and
- the shortage has the potential to have a life‑threatening or serious impact on the health of people who take, or who may need to take, the medicine.
Sponsors must notify the TGA of critical shortages within two working days of becoming aware of the shortage. Sponsors must notify the TGA of all other shortages of reportable medicines within 10 working days of becoming aware of the shortage.
Of the 1800 new shortages reported during 2019, 176 (9%) were of critical impact (Figure 3). Of these, 40% were for products on the Medicines Watch List (Figure 4).
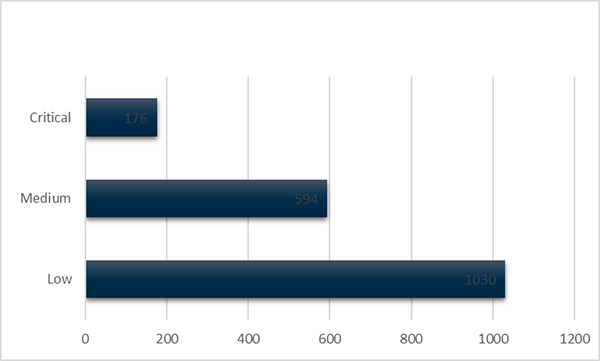
Figure 3 in table format
| Reported impact on patient | Number of notifications |
|---|---|
| Low | 1030 |
| Medium | 594 |
| Critical | 176 |

Figure 4 in text format
- Products not on the MWL: 60%
- Products on the MWL: 40%
Reasons for shortages reported by sponsors in 2019
Manufacturing issues are the most common reason (47%) given by sponsors for medicine shortages (Figure 5).

Figure 5 in table format
| Reason | Percentage |
|---|---|
| Commercial changes | 18% |
| Manufacturing | 47% |
| Other | 21% |
| Product recall | 1% |
| Unexpected increase in demand | 13% |
When reporting a shortage due to manufacturing issues, sponsors must select one of six options for the type of manufacturing issue causing the shortage. This information can assist the TGA to assess the likely length of the shortage or whether other products may be affected by the same issue. Problems during manufacture are the most commonly reported type of manufacturing issue (Figure 6).

Figure 6 in table format
| Shortage reason - Manufacturing | Number of notifications |
|---|---|
| Change in manufacturing process | 23 |
| Other | 172 |
| Problem with sourcing / importing API | 106 |
| Production problems after manufacture | 35 |
| Production problems during manufacture | 380 |
| Regulatory Delays in manufacturing site | 18 |
Shortage status
Sponsors must identify a status (anticipated, current, resolved or discontinued) in each shortage notification.
In 2019, 45% of new shortage notifications were for current shortages (Figure 7).

Figure 7 in table format
| Status | Percentage |
|---|---|
| Anticipated shortage | 36% |
| Current shortage | 45% |
| Discontinuation shortage | 19% |
Publication of notifications
The vast majority of shortage notifications are published on the TGA website. An analysis of notifications recorded in the medicine shortages database in November 2019 found that 92% of all shortage notifications had been published on the Medicines Safety Information Initiative page). Sponsors initially consented to publish 75% of notifications. Sponsors consented to publication of a further 17% of notifications following discussion with the TGA (Figure 8).
The TGA may accept sponsors' non-consent for publication if there are no compelling public health reasons for publishing. For example, a sponsor may submit a notification of an anticipated shortage to comply with reporting requirements but at the same time advise the TGA that the shortage may not eventuate pending delivery of stock. In such instances, we will liaise closely with the sponsor to monitor supply and require publication if a shortage becomes more certain. The TGA may also accept non-publication where the market share of the product in shortage is very small and other exact therapeutic alternatives are available, thereby minimising the impact of the shortage.

Figure 8 in table format
| Description | Percentage |
|---|---|
| Initially consented to publish | 75% |
| Consented to publication after consultation | 17% |
| Not published | 8% |
Section 19A approvals
Section 19A of the Act allows for the supply of medicines not currently included in the ARTG in place of a registered medicine that is unavailable or in short supply. Section 19A approvals are granted in the interests of public health and are subject to relevant conditions to minimise risks to consumers. The TGA may consider supply of a medicine under Section 19A if it is registered or approved for general marketing in a specified country[1]. If medicines from the specified countries are not available to address the shortage, the TGA may consider approving supply of a medicine from another country if the manufacturing and quality control procedures used in the production of the medicine are acceptable.
The TGA assesses each Section 19A application on a case-by-case basis and minimises risk by considering:
- the clinical need for an uninterrupted supply of the medicine and whether suitable therapeutic alternatives on the ARTG are available
- the projected demand for the medicine above current supply
- conditions of supply to mitigate risks; and
- duration of supply.
The number of Section 19A approvals granted by the TGA increased by 40% during 2019 compared with 2018 (Figure 9).

Figure 9 in table format
| Year | Number |
|---|---|
| 2015 | 34 |
| 2016 | 38 |
| 2017 | 58 |
| 2018 | 82 |
| 2019 | 113 |
In 2019, the TGA approved 113 of 195 applications submitted for supply under Section 19A. During the assessment process, the TGA works with the relevant sponsor(s) to determine whether there is a confirmed shortage and that enough information has been provided to undertake a risk assessment to ensure that the risks to the consumer are minimised. Applications may not proceed for a number of reasons including:
- after a TGA market assessment, it is determined that the product is not in shortage
- the shortage has been mitigated by approval of a Section 19A application from another sponsor
- the Section 19A application is incomplete or does not meet the Section 19A criteria (for example, Good Manufacturing Practice (GMP) documentation is incomplete or not provided following repeated requests).
Enhancements to the medicine shortages scheme
The Medicine Shortages Working Party was convened to develop the protocol for the management and communication of medicine shortage notifications leading to the introduction of the mandatory reporting scheme in 2019. The Working Party includes representatives of the Pharmacy Guild, Pharmaceutical Society of Australia, Generic and Biosimilar Medicines Association, Medicines Australia, Australian Medical Association, Society of Hospital Pharmacists of Australia, Consumer Healthcare Products Australia and National Pharmaceutical Services Association and is chaired by the Department of Health.
The Working Party last met on 11 December 2019 to review the performance of the mandatory reporting scheme and address other issues relating to medicine shortages.
The Working Party agreed on a range of actions to improve shortage reporting, communications, mitigation and arrangements for supply of alternative products.
These actions include:
- Publishing this report summarising the first 12 months of mandatory reporting of medicine shortages
- Improving the TGA's ability to anticipate shortages through exploring opportunities to use sponsor supply and manufacturing data and identifying supply chain issues that lead to medicine shortages and delays in reporting
- Improving public access to information about shortages by increasing the proportion of shortage notifications that are published on the TGA website and implementing communications activities for healthcare professionals
- Supporting rapid mitigation of shortages by clarifying guidance for sponsors about Section 19A application requirements and ensuring good coordination between relevant areas of the Department of Health.
The TGA is currently working with members of the working party to progress these action items and will report their progress at the next meeting planned in April 2020.
Footnotes
| [1] | The currently specified countries are Canada, France, Germany, Netherlands, New Zealand, Sweden, Switzerland, United Kingdom and United States of America. |
|---|
| Version | Description of change | Author | Effective date |
|---|---|---|---|
| V1.0 | Original publication | Risk Management Section/Pharmacovigilance and Special Access Branch | February 2020 |

