This is a step-by-step guide for agents and sponsors who wish to apply for an extension of a previously approved provisional determination and/or Orphan drug designation of a prescription medicine. Priority review determinations cannot be extended.
If you are logging in as a sponsor, a number of fields that are visible to agents will not be visible to you. This is because we already hold certain information about you and do not need you to re-enter it.
Before you apply
To make an application you will need:
- a TGA client ID number
- access to the TGA Business Services (TBS) portal
If you do not have a client ID number or access to the TGA Business Services portal, go to TGA Business Services: getting started with TGA and submit the online organisation details form.
Completing your application
- Log in to TGA Business Services.
From the Applications dropdown menu, go to Prescription Medicine and select Designation/Determination Extension.

- An application window will open. This window offers one tab:
Designation/determination Extension Application Details
This tab contains a number of fields to fill in as well as details of information that you provided as part of the original determination or designation application.
Fields marked with a red asterisk (*) are always mandatory.
Saving your draft application
If you need to exit your application before it is complete, you can save it as a draft.
From within the application screen, click on the Save button at the bottom right of your browser window.

Your changes will be saved as a draft. You can now close your application.
When you next log in to your Business Portal, go to the My work menu and click on the arrow beside Work on drafts.

This will open a list of your drafts.
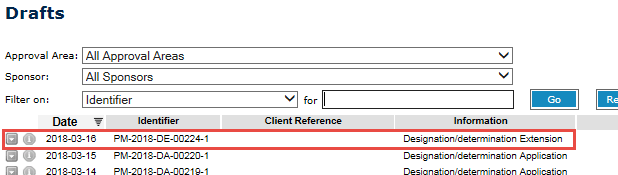
- Double-click on the Designation/determination Extension application to reopen your draft and continue.
Applicant Details heading
Enter the applicant details under the Applicant Details heading.
The first field on this tab is Applicant name. This is a mandatory field and will be automatically populated based on your login details. If you are an agent, go to Step 2 as you will need to select your client Sponsor organisation.

If you are logged in as a sponsor, you do not need to complete step 2 or Step 4.
The next field is Sponsor organisation. This field is mandatory. Click on the arrow at the right of the field and select the appropriate sponsor organisation from the dropdown list.

Select the appropriate correspondence address from the drop down menu

Next, indicate whether you wish us to send fee invoice to sponsor. Select either the Yes or No radio button as appropriate.

Select the correct Billing address from the dropdown menu.

The next field allows you to select a Primary contact person. Click on the arrow at the right of the field to select the appropriate contact person from the dropdown list.
The Name, Telephone number and Email fields will auto-populate once you have made your selection.

If you are logged in as a sponsor, these fields will already be populated. You can change the auto-populated details by clicking on the Clear button at the right of the Primary contact person field.
Finally, you may also nominate a Secondary contact person. This field is not mandatory.
Click on the down arrow at the right of the field to select from the dropdown list. The Name, Telephone number and Email fields will auto-populate once you have made your selection.

Designation/determination Application Details heading
The Designation/determination Application Details heading offers explanatory information on which designations/determinations are eligible for extension.
Select the Eligible Designation/determination submission from the dropdown list.
Submissions will only display in this list if they are eligible for extension i.e. they relate to Provisional determination or Orphan drug designation, have at least 28 days remaining until the lapse date, and have not lapsed, been extended or used.

Select the relevant Extension Option(s). The options that are available to select depend on the designation/determination that is linked to the submission that you selected in the previous question i.e. if the submission related to Provisional determination only, you will only be able to select the Extend Provisional determination option.
If the submission you selected contained both a valid Provisional determination and an Orphan drug designation, you can choose to extend only one or both. If you only extend one, you can come back and extend the other designation/determination linked to that submission number by creating a separate extension application.

If you submitted a combined orphan drug designation and provisional determination, Selecting the relevant combined submission will allow you to Extend both.

The next field asks you to indicate whether the product in your application has already been designated as an Orphan drug for the proposed indication. Select either Yes or No as appropriate.

If you wish to select No, you must first click on Yes and then click on No to auto-populate the Determination extension fee field.
The next field is Determination extension fee. If you select Yes for the previous question, the field will auto-populate with $0.00. If you select No, the field will auto-populate with the appropriate fee.
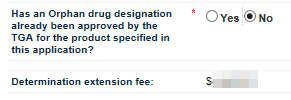
There is no fee for an Orphan drug designation extension application. If you are applying for extension of both Provisional determination and Orphan drug designation, you are eligible for orphan drug waiver and will therefore not be charged the extension fee. If you are only applying for extension of a Provisional determination only, then the Determination Extension Fee will be charged.
Provisional Determination extension
Selecting Extend Provisional determination will open explanatory text and a number of related fields:
- New medicine
- Serious condition
- Comparison against existing therapeutic products
- Major therapeutic advance
- Clinical study plan
- Application for provisional registration
These fields are mandatory. Select an answer for each field in line with the guidance provided.

Orphan Drug Designation Extension
Selecting Extend Orphan Drug designation will open explanatory text and a number of related fields:
- Previous extension
- Overseas regulators and safety
- Comparison with registered therapeutic goods
- Application for inclusion in the Australian Register of Therapeutic Goods
These fields are mandatory. Select an answer for each field in line with the guidance provided.

The same extension criteria apply, regardless of whether the orphan drug designation is for a new dosage form medicine or not.
Upload Supporting Documentation heading
The Upload Supporting Documentation section offers explanatory information on which designations/determinations are eligible for extension.
- You can upload supporting documentation which will be displayed in the table. See our guidance on eligibility criteria and supporting documentation for Provisional determination and Orphan drug designation for more information on what documentation should be provided to support your designation/determination extension application.
The Add and Remove buttons will be greyed out until you have saved the form at least once. To activate these buttons, click on the Save button at the bottom right of your window.

Once you have saved the document, the Add and Remove buttons will become active. Click on the Add button to upload a supporting document.
This will open an Attachment Details pop-up window.

Both fields in the Attachment Details pop-up window, Description and Supporting Document, are mandatory. Enter a description of the file and then click on the Browse button at the right of the Supporting Document field to find and upload the document.
- Click on Save & Close to upload your file.
Any file you upload must meet the formatting requirements outlined in parts A and B of the general dossier requirements.
Upload separate documents for each determination and designation you are applying for.
Original designation/determination submission details
Clicking on the arrow to the left of Original designation/determination submission details will open up a section of the form which displays the information that you provided as part of the original designation/determination application form for the selected submission.

This information cannot be edited. If any of these details have changed since your original designation/determination application and you have not already provided an update to the TGA, provide this information in the supporting documentation attached to the designation/determination extension application form.
Validating your application
After you have saved the form, the Validate radio button will become active. You must validate your application before you can submit it.
Validating
Click on the Validate button at the bottom right of your browser window.

This will trigger a pop-up window.

- If there are any issues with your application, a new pane will open on the right of your browser window. The issues will be listed in this pane.
If you double-click on a listed validation issue in this pane, it will open your application at the appropriate tab so you can rectify the issue. (This screenshot shows example messages only. You may have no messages or different messages).
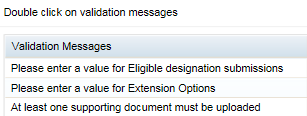
When you have rectified all validation issues, click validate again. Once validation is successful, the Submit button at the bottom right of your browser window will become active.

You are now ready to submit your application.
Submitting your application
When you are ready to submit your application, click on the Submit button at the bottom right of the browser window. This will open a Declaration pop-up window.
Declaration
Check the details in the declaration. If the details are correct and you agree with the acknowledgements, click on the Agree button at the bottom left of the pop-up.

Your submission will be lodged and you will be assigned a submission number.

You can close the completed application by clicking on the Close button at the bottom right of your browser window.
Application assessment
For more information on how we will assess your application(s), see the Provisional determination or Orphan drug designation step-by-step guides.
If you have read the guidance and still require assistance, contact: AET.Application.Entry.Team@health.gov.au.
Version history
| Version | Description of change | Author | Effective date |
|---|---|---|---|
| V1.0 | Original publication | Prescription Medicines Authorisation Branch and Regulatory Guidance Team | March 2018 |

