3. Delegate-only decisions on agricultural and veterinary chemicals
On this page: Delegate's final decision | Applicant's scheduling proposal and reasons for proposal | Current scheduling status | Scheduling history | Australian regulations | International regulations | Substance summary
Delegate's final decision
The delegate's final decision under regulation 42ZCZU of the Therapeutic Goods Regulations 1990 (the Regulations) is to amend the Poisons Standard by creating a new Schedule entry for benzovindiflupyr as follows:
Note
New text is shown as green, larger font, with a horizontal line above it.
Schedule 6 - New Entry
BENZOVINDIFLUPYR
Index - New Entry
BENZOVINDIFLUPYR
Schedule 6
Implementation date: 1 February 2019
Delegate's considerations
The delegate considered the following in regards to this proposal:
- The application to amend the current Poisons Standard with respect to Benzovindiflupyr
- Section 52E (1) of the Therapeutic Goods Act 1989 in particular: (a) the risks and benefits of the use of a substance; (b) the purposes for which a substance is to be used and the extent of use of a substance; and (c) the toxicity of a substance.
- The Australian Health Ministers' Advisory Council's Scheduling Policy Framework (SPF 2018)
Reasons:
The matters under subsection 52E (1) of the Therapeutic Goods Act 1989 considered relevant by the delegate for the decision include:
- the risks and benefits of the use of a substance:
Benzovindiflupyr is a broad spectrum foliar fungicide belonging to the succinate dehydrogenase inhibitor (SDHI) pyrazole carboxamide class. As a class, SDHI display a high selectivity for fungal mitochondrial complex II, with mammalian mitochondrial complex II being insensitive to the effects of these fungicides.
The risks of the proposed use of benzovindiflupyr are its moderate acute oral and inhalation toxicity and that it is moderately irritating to the eye.
- the purposes for which a substance is to be used and the extent of use of a substance:
Benzovindiflupyr has not been previously considered for scheduling. It is proposed to be used in an agricultural chemical product in Australia. It is a broad spectrum foliar fungicide belonging to the succinate dehydrogenase inhibitor (SDHI) pyrazole carboxamide class.
Benzovindiflupyr is approved for use in certain EU countries (100 g/L formulations) for the control of certain fungal diseases of wheat and barley. It has conditional registration in the US for use in a broader range of crops including corn, blueberries, cucurbit vegetables, cotton seed, legume vegetables, pome fruit, fruiting vegetables, turf and ornamentals. The overseas registrations are for a variety of other formulations and combinations with or without other active constituents, including propiconazole, prothioconazole, azoxystrobin, and difenoconazole.
- the toxicity of a substance:
Benzovindiflupyr has moderate acute and inhalation toxicity and low acute dermal toxicity. It is a moderate eye irritant but not a skin irritant or sensitiser. It is not neurotoxic, genotoxic, carcinogenic, teratogenic or immunotoxic. The toxicity profile of benzovindiflupyr therefore supports inclusion in Schedule 6.
- the dosage, formulation, labelling, packaging and presentation of a substance:
Nil
- the potential for abuse of a substance:
Nil
- any other matters that the Secretary considers necessary to protect public health:
Nil
Applicant's scheduling proposal and reasons for proposal
An application was submitted by the Australian Pesticides and Veterinary Medicines Authority (APVMA) to create a new Schedule 6 entry for 'benzovindiflupyr' in the Standard for the Uniform Scheduling of Medicines and Poisons (SUSMP) – the Poisons Standard.
The applicant's reasons for the request are:
- The available toxicological data for benzovindiflupyr is considered to be sufficient for the purposes of recommending a scheduling decision.
- From the available data and international assessment reports, benzovindiflupyr has moderate acute oral and inhalation toxicity and low acute dermal toxicity. It is a moderate eye irritant but not a skin irritant or sensitiser. It is not neurotoxic, genotoxic, carcinogenic, teratogenic or immunotoxic. The toxicity profile of benzovindiflupyr supports consideration for listing in Schedule 6.
- Benzovindiflupyr, a fungicide, is approved for use in the EU and has conditional registration in the US. However, the formulated product proposed for registration in Australia has not been considered overseas. The EU considered an EC formulation (A15457H) containing 100 g/L benzovindiflupyr. Other formulations and combinations with/without a second active constituent have been registered overseas.
Current scheduling status
Benzovindiflupyr is not specifically scheduled in the current Poisons Standard and has not previously been considered for scheduling.
Scheduling history
Benzovindiflupyr is not currently scheduled and has not been previously considered for scheduling. Therefore a scheduling history is not available.
Australian regulations
Benzovindiflupyr is not currently approved in Australia. The proposed product in Australia is an emulsifiable concentrate formulation containing the new active benzovindiflupyr (40 g/L) and the currently approved active propiconazole (250 g/L).
Benzovindiflupyr is not listed on the Therapeutic Goods (Permissible Ingredients) Determination No. 3 of 2018 - external site.
Benzovindiflupyr is neither an excipient nor active in any medicines on the ARTG.
International regulations
Benzovindiflupyr is approved for use in certain EU countries for the control of certain fungal diseases of wheat and barley. Conditional registration is established in the US with use in a broader range of crops including corn, blueberries, cucurbit vegetables, cotton seed, legume vegetables, pome fruit, fruiting vegetables, turf and ornamentals. The product proposed for registration in Australia is not currently registered elsewhere. Overseas registration is for a variety of other formulations, with or without other active constituents, including propiconazole, prothioconazole, azoxystrobin, and difenoconazole.
Substance summary
Benzovindiflupyr (ISO common name) is a broad spectrum foliar fungicide belonging to the succinate dehydrogenase inhibitor (SDHI) pyrazole carboxamide class.
| Property | Benzovindiflupyr | |
|---|---|---|
|
Chemical structure (showing stereochemistry) |
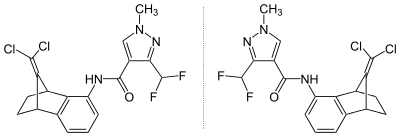 |
|
|
SYN546526 N-[(1S,4R)-9-(dichloromethylene)-1,2,3,4-tetrahydro-1,4-methanonaphthalen-5-yl]-3-(difluoromethyl)-1-methylpyrazole-4-carboxamide |
SYN546527 N-[(1R,4S)-9-(dichloromethylene)-1,2,3,4-tetrahydro-1,4-methanonaphthalen-5-yl]-3-(difluoromethyl)-1-methylpyrazole-4-carboxamide |
|
|
Molecular weight |
398.2 g/mol |
|
|
Molecular formula |
C18H15Cl2F2N3O |
|
|
CAS names |
1072957-71-1 |
|
|
IUPAC and/or common and/or other names |
IUPAC: N-[(1RS,4SR)-9-(dichloromethylene)-1,2,3,4-tetrahydro-1,4-methanonaphthalen-5-yl]-3-(difluoromethyl)-1-methylpyrazole-4-carboxamide; CAS: 1H-pyrazole-4-carboxamide, N-[9-(dichloromethylene)-1,2,3,4- tetrahydro-1,4-methanonaphthalen-5-yl]-3-(difluoromethyl)-1- methyl Other: solatenol, benzovindiflupyr PubChem CID: 51347655; EC No. EC 691-719-4 |
|
| Toxicity | Species | Benzovindiflupyr | SPF (2018) Classification |
|---|---|---|---|
|
Acute oral toxicity LD50 (mg/kg bw) |
Rat |
55 |
6 |
|
Acute dermal toxicity LD50 (mg/kg bw) |
Rat |
>2000 |
5 |
|
Acute inhalational toxicity LC50 (mg/m3/4 h) |
Rat |
>560 |
6 |
|
Skin irritation |
Rabbit |
Non-irritant* |
- |
|
Eye irritation |
Rabbit |
Moderate irritant |
5 |
|
Skin sensitisation (LLNA) |
Mouse |
Non-sensitising |
- |
*JMPR classifies this as a mild irritant, GHS Category 3, based on slight erythema at 1 and 24 h observations. This category is not recognised by the EU, who classified this as non-irritant. APVMA classifies this as non-irritating, based on no dermal effects persisting until 72 hours.
Toxicity studies on benzovindiflupyr have been submitted to the APVMA in support of the active approval and have been reviewed by JMPR (2013)[5] and EFSA (2015),[6] the latter based on the EFSA Draft Assessment Report (2014). Studies on the product proposed to be registered in Australia have been submitted and were not considered by JMPR or EFSA. These studies will be assessed by the APVMA as part of the product evaluation at a later date.
Acute toxicity
Studies in rats done according to OECD guidelines show that benzovindiflupyr has moderate acute oral toxicity, low acute dermal toxicity, and moderate inhalation toxicity.
Skin and eye irritation
OECD guideline-compliant studies in rabbits show that benzovindiflupyr is not a skin irritant but is a moderate eye irritant.
Sensitisation
OECD guideline-compliant studies shows that benzovindiflupyr is not a skin sensitiser in the mouse local-lymph node assay (LLNA).
Repeat-dose toxicity
In repeat-dose oral toxicity studies in mice, rats and dogs, the main effects were on body weight and food consumption with minor findings in the liver. Repeat-dose dermal application resulted in no toxicity.
- In 28 and 90 day dietary studies in mice, the NOAEL was 100 ppm (16-17 mg/kg bw/d) based on body weight loss/reduced body weight gain at 300 ppm or higher.
- In 28 and 90 day dietary study in rats, the NOAEL was 100 ppm (9 mg/kg bw/d in the 28 day study and 7.6-8.2 mg/kg bw/d in the 90 day study) based on reduced body weight/body weight gain and food consumption at 400 ppm in the 28-d study and 750 ppm in the 90-d study.
- Dogs receiving doses up to 750 mg/kg bw/d in capsules for 13 weeks showed minimal findings from 375 mg/kg bw/d ( reduced food consumption, loss of body weight/reduced body weight gain and minor clinical chemistry). The NOAEL was 30 mg/kg bw/d based on a >10% reduction in body weight gain.
- Repeat dose dermal application of benzovindiflupyr to rats for 28-d showed no systemic or local effects. The NOAEL was 1000 mg/kg bw/d, the highest dose tested.
- In an 80-week dietary study in mice, the NOAEL was 60 ppm (7.6 (M) and 8.7 (F) mg/kg bw/d) based on a LOAEL of 200pm for reduced body weight gain and an increased incidence of mucosal hyperplasia in the large intestine.
- In a 104-week dietary study in rats, the NOAEL was 100 ppm (4.9 (M) and 6.7 (F) mg/kg bw/d) based on a LOAEL of 400 (M) and 600 (F) mg/kg bw/d for reduced body weight gain and food consumption, and hepatic toxicity.
- In a 2-year dog study, capsule administration of benzovindiflupyr established a NOAEL of 250 mg/kg bw/d based on reduced body weight gain at 500 mg/kg bw/d.
Neurotoxicity
Neurotoxicity studies in rats were conducted as a single oral gavage dose of up to 10 mg/kg bw/d and dietary dosing over 90-days at doses up to 38 mg/kg bw/d. Benzovindiflupyr was not neurotoxic.
Carcinogenicity
Chronic studies in mice (diet; up to 200 ppm equal to 26 (M) and 29 (F) mg/kg bw/d), rats (diet; up to 400/600 ppm for F/M, equal to 30 (M) and 27 (F) mg/kg bw/d) and dogs (capsule; up to 500 mg/kg bw/d) demonstrated benzovindiflupyr is not carcinogenic. Tumours noted in rodent studies were determined not to be relevant to humans.
Genotoxicity
A range of in vivo and in vitro assays for genotoxicity indicated benzovindiflupyr is unlikely to be genotoxic.
Reproduction and developmental toxicity
No reproductive toxicity of benzovindiflupyr was observed in a multi-generation reproduction dietary study in rats at doses up to 250 ppm for females and 600 ppm for males. The parental and offspring NOAEL was 100 ppm (equal to 6.8 mg/kg bw/d for P generation males during pre-pairing and 7.8 mg/kg bw/d for F1 males during pre-pairing) based on reduced body weight gain and food consumption, liver effects (adaptive/mild toxicity) and lower postnatal pup weights at 250 ppm in females (equivalent to 19.4 mg/kg bw/d for P generation females during pairing) and 600 ppm males (equal to 40.5 mg/kg bw/d for P generation males during pre-pairing). The NOAEL for direct effects on reproduction was the highest dose tested (250 ppm).
In an oral gavage developmental toxicity studies in rats and rabbits, benzovindiflupyr was not teratogenic. In rats, the maternal and foetal NOAEL was 15 mg/kg bw/d based on clinical signs, reduced food intake and body weight in dams and reduced weight and delayed ossification in foetuses at doses toxic to dams. The developmental NOAEL was 30 mg/kg bw/d, the highest dose tested. In rabbits, NOAEL for maternal, fetal and developmental effects was 35 mg/kg bw/d, the highest dose tested.
Immunotoxicity
Benzovindiflupyr did not show immunotoxic potential in a 28-day immunotoxicity study in mice at doses up to 400 ppm (equals 97 mg/kg bw/d) in the diet.
Summary
From the available data and the JMPR and EFSA12, 13 assessment reports, benzovindiflupyr has moderate acute oral and inhalational toxicity and low acute dermal toxicity. It is a moderate eye irritant but neither a skin irritant nor skin sensitiser. Health effects in animals given repeated doses primarily involved decreased body weight and body weight gain, effects on the liver and indications of general toxicity. It is not neurotoxic, genotoxic, carcinogenic, teratogenic or immuno-toxic. The toxicity profile of benzovindiflupyr supports consideration for listing in Schedule 6.

