We will have limited operations from 15:00 Tuesday 24 December 2024 (AEDT) until Thursday 2 January 2025. Find out how to contact us during the holiday period.
Part A - Interim decisions on matters referred to an expert advisory committee (November 2016)
2. Joint meeting of the Advisory Committee on Chemicals and Medicines Scheduling (ACCS-ACMS #14)
2.3 Cannabis
Referred scheduling proposal
The medicines scheduling delegate in view of the upcoming rescheduling of cannabis and tetrahydrocannabinols (THCs) proposes to consider:
The final decision for cannabis provides for hemp seed oil to be exempt from Schedules 8 and 9 when the levels of total cannabinoids are 50 mg/kg or less.
Due to further information provided after the publication of the final decision, the scheduling delegate is undertaking further consideration. This proposal seeks to determine whether this cut-off for total cannabinoids is appropriate for hemp seed oil, and the delegate is requesting additional information on the levels of cannabinoids (including tetrahydrocannabinols) in hemp seed oil. The delegate is also proposing to add to the cannabis entries regarding the hemp seed oil exception the following:
"when labelled with either of the following warning statements:
- Not for internal use; or
- Not to be taken."
Current scheduling status
Cannabis and cannabinoids are currently listed in Schedules 4, 8 and 9, and in Appendix D and Appendix K.
Hemp seed oil is defined in the Interpretation of the Poisons Standard as follows:
PART 1 - INTERPRETATION
"Hemp seed oil" means the oil obtained by cold expression from the ripened fruits (seeds) of Cannabis sativa.
Following the 31 August 2016 final scheduling decision for cannabis and tetrahydrocannabinols to be implemented on 1 November 2016, the entries for cannabis, tetrahydrocannabinols and nabiximols will be listed in Schedules 8 and 9, and Appendix D and Appendix K as follows:
Schedule 9
CANNABIS (including seeds, extracts, resins, and the plant and any part of the plant when packed or prepared), except:
- when separately specified in these Schedules; or
- processed hemp fibre containing 0.1 per cent or less of tetrahydrocannabinols, and hemp fibre products manufactured from such fibre; or
- in hemp seed oil for purposes other than internal human use containing 50 mg/kg or less of cannabinoids.[6]
TETRAHYDROCANNABINOLS and their alkyl homologues, except:
- when included in Schedule 4 or Schedule 8; or
- processed hemp fibre containing 0.1 per cent or less of tetrahydrocannabinols, and hemp fibre products manufactured from such fibre; or
- in hemp seed oil containing 50 mg/kg or less of tetrahydrocannabinols when labelled with either of the following warning statements:
- Not for internal use; or
- Not to be taken; or
- in products for purposes other than internal human use containing 50 mg/kg or less of tetrahydrocannabinols.
Schedule 8
CANNABIS (including seeds, extracts, resins and the plant, and any part of the plant) when prepared or packed for human therapeutic use, when:
- cultivated or produced, or in products manufactured[7], in accordance with the Narcotic Drugs Act 1967; and/or
- for use in products manufactured in accordance with the Narcotic Drugs Act 1967; and/or
- imported as therapeutic goods, or for use in therapeutic goods, for supply, in accordance with the Therapeutic Goods Act 1989; and/or
- in therapeutic goods supplied in accordance with the Therapeutic Goods Act 1989,
- except when:
- it is a product to which item 4, 8, 10, 11 or 12 of Schedule 5A to the Therapeutic Goods Regulations 1990 applies; or
- separately specified in Schedule 4; or
- separately specified in the NABIXIMOLS entry in this Schedule; or
- in hemp seed oil for purposes other than internal human therapeutic use containing 50 mg/kg or less of cannabinoids.
TETRAHYDROCANNABINOLS when extracted from cannabis for human therapeutic use, when:
- included in products manufactured in accordance with the Narcotic Drugs Act 1967; and/or
- imported as therapeutic goods, or for use in therapeutic goods, for supply, in accordance with the Therapeutic Goods Act 1989; and/or
- in therapeutic goods supplied in accordance with the Therapeutic Goods Act 1989,
- except when:
- it is a product to which item 4, 8, 10, 11 or 12 of Schedule 5A to the Therapeutic Goods Regulations 1990 applies; or
- in hemp seed oil, containing 50 mg/kg or less of tetrahydrocannabinols when labelled with either of the following warning statements:
- Not for internal use; or
- Not to be taken; or
- in products for purposes other than for internal human use containing 50 mg/kg or less of tetrahydrocannabinols; or
- separately specified in the NABIXIMOLS entry in this Schedule.
NABIXIMOLS (botanical extract of Cannabis sativa which includes the following cannabinoids: tetrahydrocannabinols, cannabidiol, cannabinol, cannabigerol, cannabichromene, cannabidiolic acid, tetrahydrocannabinolic acids, tetrahydrocannabivarol, and cannabidivarol, where tetrahydrocannabinols and cannabidiol (in approximately equal proportions) comprise not less than 90 per cent of the total cannabinoid content) in a buccal spray for human therapeutic use.
Schedule 4
CANNABIDIOL in preparations for therapeutic use containing 2 per cent or less of other cannabinoids found in cannabis.
Appendix D, Item 1
CANNABIS for human use.
TETRAHYDROCANNABINOLS for human use.
Appendix K
CANNABIS
TETRAHYDROCANNABINOLS
Index
CANNABICHROMENE
cross reference: NABIXIMOLS, CANNABIS
CANNABIDIOL
cross reference: NABIXIMOLS, CANNABIS
CANNABIDIOLIC ACID
cross reference: NABIXIMOLS, CANNABIS
CANNABIDIVAROL
cross reference: NABIXIMOLS, CANNABIS
CANNABIGEROL
cross reference: NABIXIMOLS, CANNABIS
CANNABINOIDS
cross reference: NABIXIMOLS, CANNABIS, TETRAHYDROCANNABINOLS
CANNABINOL
cross reference: NABIXIMOLS, CANNABIS
CANNABIS
cross reference: CANNABIS SATIVA, HEMP, HEMP SEED OIL, TETRAHYDROCANNABINOLS
TETRAHYDROCANNABINOLIC ACID
cross reference: NABIXIMOLS, TETRAHYDROCANNABINOLS
TETRAHYDROCANNABINOLS
cross reference: CANNABIS, HEMP SEED OIL, NABIXIMOLS
TETRAHYDROCANNABIDIVAROL
cross reference: NABIXIMOLS, TETRAHYDROCANNABINOLS
Relevant scheduling history
CANNABIS
In August 1999, the committee reviewed the status of its foreshadowed proposal to amend the Schedule 9 entry for cannabis to exempt from scheduling cannabis when grown commercially for fibre production and manufactured goods containing hemp fibre. It was seen that such a proposal would provide uniformity in controls exerted by state and territory governments. A general exemption for hemp fibre and hemp fibre products could be made. The committee considered a general exemption for hemp fibre and hemp fibre products could be made. The exemption would allow sale of such hemp fibre and manufactured products in all jurisdictions.
TETRAHYDROCANNABINOLS
In May 1998, the committee considered additional technical and regulatory information relating to a request to exempt from Schedule 9 tetrahydrocannabinols when in hemp seed oil and products for external use when containing 50 mg/kg or less of tetrahydrocannabinols (THC). The committee supported the proposal that hemp seed oil and products containing hemp seed oil should be exempt from the Schedule 9 entry for tetrahydrocannabinols when containing 50 mg/kg of THC and when for external use.
NABIXIMOLS (SATIVEX®)
In October 2009, the committee considered an entry specific for Cannabis sativa extract, nabiximols, after the issue was raised at the June 2009 meeting that certain jurisdictions were unable to allow SAS access to the substance as it was captured under Schedule 9. As discussed in June, the committee members agreed on the Schedule 8 listing. The committee also agreed that the Schedule 8 entry should limit the allowed presentation to buccal sprays as this would further reinforce the very restricted scope of this entry and would require any new presentation to be brought to the attention of the committee.
In May 2010, nabiximols were included in Schedule 8 and Appendices D and K. The committee advised that nabiximols needed to be added to Appendix D, Item 3 to limit access through SAS Category A. This addition would allow restricted access to nabiximols only, not to cannabis extracts, but would not prohibit use for clinical trials provided by an authorised prescriber only. The committee agreed to not restrict the Schedule 8 nabiximols entry by indication (for Multiple Sclerosis). Members additionally agreed that it would be appropriate to include nabiximols in Appendix K due to sedating effects.
In March 2013, the committee considered a proposal to reschedule nabiximols from Item 3 of Appendix D to Item 1 of Appendix D of the SUSMP and amended Appendix D to include the entry of nabiximols.
In August 2016, the delegate amended the nabiximols entry in line with the August 2016 decision for cannabis and tetrahydrocannabinols to use the plural 's' for tetrahydrocannabinols and their acids.
CANNABIS AND TETRAHYDROCANNABINOLS
In March 2016, the committee considered a proposal to amend the Schedule 9 entries and create new Schedule 8 entries for cannabis and tetrahydrocannabinols with Appendix D, Part 1 and Appendix K warnings. The committee supported the proposal and in August 2016, the Medicines Scheduling Delegate decided to amend the scheduling entries for cannabis, tetrahydrocannabinols and nabiximols, with an implementation date of 1 November 2016.
Scheduling application
This was a delegate-initiated application. The delegate’s proposed amendments to the Poisons Standard are as follows:
Schedule 9 - Amend entry
CANNABIS (including seeds, extracts, resins, and the plant and any part of the plant when packed or prepared), except:
- when separately specified in these Schedules; or
- processed hemp fibre containing 0.1 per cent or less of tetrahydrocannabinols, and hemp fibre products manufactured from such fibre; or
- in hemp seed oil for purposes other than internal human use containing X mg/kg or less of cannabinoids when labelled with either of the following warning statements:
- Not for internal use; or
- Not to be taken.
Schedule 8 - Amend entry
CANNABIS (including seeds, extracts, resins and the plant, and any part of the plant) when prepared or packed for human therapeutic use, when:
- cultivated or produced, or in products manufactured[8], in accordance with the Narcotic Drugs Act 1967; and/or
- for use in products manufactured in accordance with the Narcotic Drugs Act 1967; and/or
- imported as therapeutic goods, or for use in therapeutic goods, for supply, in accordance with the Therapeutic Goods Act 1989; and/or
- in therapeutic goods supplied in accordance with the Therapeutic Goods Act 1989,
- except when:
- it is a product to which item 4, 8, 10, 11 or 12 of Schedule 5A to the Therapeutic Goods Regulations 1990 applies; or
- separately specified in Schedule 4; or
- separately specified in the NABIXIMOLS entry in this Schedule; or
- in hemp seed oil for purposes other than internal human therapeutic use containing X mg/kg or less of cannabinoids when labelled with either of the following warning statements:
- Not for internal use; or
- Not to be taken.
Appendix D, Item 1
CANNABIS for human use.
Appendix K
CANNABIS
The applicant's reasons for the request are:
- Currently hemp seed oil has no restriction on cannabinoid content other than 50 mg/kg or less of tetrahydrocannabinols when labelled with either of the following warning statements:
- Not for internal use; or
- Not to be taken
Australian regulatory information
Narcotics Drugs Act and importation
Under the Narcotic Drugs Act 1967 (the ND Act) a 'drug' includes all extracts of cannabis (including hemp) from cannabis plants.
The manufacture of a drug that includes (or is from) a cannabis plant, can only be authorised under a manufacture licence in limited circumstances under the ND Act. As outlined under Section 11K, the Secretary must refuse to grant a manufacture licence if not satisfied on reasonable grounds with one of the following (these are set out in paragraphs 11K(2)(b) and (c), respectively):
- that the drug is a medicinal cannabis product that will be:
- supplied for the purposes of use in a clinical trials that is, or is likely to be approved under the Therapeutic Goods Act 1989 (the TG Act) or notified to the Secretary under that Act; or;
- otherwise supplied in accordance with an approval or authority under the TG Act; or
- supplied by a pharmacist in a public hospital in accordance with the TG Act;
- that the drug is a medicinal cannabis product that is registered within the meaning of the TG Act under section 25 of that Act.
Therefore extracts of cannabis (or hemp), or the manufacture of drugs from cannabis plants, may only be for the purposes of the aforementioned activities.
Extracts for food, cosmetics, veterinary use (including pet food) are not permitted.
Cannabis, cannabinoids, cannabis resins, tetrahydrocannabinols, cannabis seeds, cannabis plants and parts of cannabis plants are prohibited imports under the Customs (Prohibited Imports) Regulations 1956. Cannabis and THCs that are in Schedule 9 will not be granted an import permit, unless a State or Territory Health Department agency also authorises/grants the applicant a permission to possess, hold or supply cannabis or THC listed under Schedule 9 of the current Poisons Standard.
Substance summary
Cannabis is a term used to describe a range of varieties of the Cannabis genus. The Cannabis plant produces a resin containing compounds called cannabinoids. Some cannabinoids possess psychoactive properties.
Cannabis contains about 60 cannabinoids, of which the main active constituent is delta-9-tetrahydrocannabinol. Delta-9-tetrahydrocannabinol reportedly has anti-emetic properties and has been associated with claims relating to use for the control of nausea and vomiting associated with cancer chemotherapy in patients who have failed to respond adequately to conventional anti-emetics. Another active cannabinoid present in Cannabis is cannabidiol that is associated with claims relating to use as an analgesic, anticonvulsant, muscle relaxant, anxiolytic, neuroprotective, anti-oxidant and anti-psychotic.
Nabiximols is a specific extract of Cannabis sativa which contains a range of cannabinoids, of which tetrahydrocannabinols and cannabidiol in approximately equal proportions comprise not less than 90% of the total cannabinoid content. Nabiximols are registered for use in Australia as a buccal spray preparation (Sativex®) as an adjunctive treatment for the symptomatic relief of neuropathic pain in multiple sclerosis in adults.
Nabilone is a synthetic cannabinoid used as an anti-emetic in the treatment of nausea and vomiting caused by chemotherapy and also for patients who are not responsive to conventional anti-emetic treatments.
Hemp seed oil as defined in Part 1 Interpretation, Paragraph (1) of the Poisons Standard is the oil obtained by cold expression from the ripened fruits (seeds) of Cannabis sativa. Hemp oil, is distinct from hemp seed oil and includes extracts from the flowering tops or leaves or any other part of the Cannabis plant other than the ripened fruit (seeds).
Information in the public domain, including websites and literature articles[9] report cannabinoids are not synthesised within the hemp seed. However, traces of delta-9-tetrahydrocannabinol and cannabidiol contamination of the seed may occur due to residual contamination of the outside of the seed coat, even under good agricultural/manufacturing practice. Rigorous cleaning methods, including washing, sieving and shelling, may help reduce or remove any cannabinoid contamination of seeds.
Reported gas chromatography (GC) analytical composition data from literature (Leizer, et al, 2000) determined for hemp seed oil (variety Fedora-19) is below. The reported composition includes significant portions of polyunsaturated fatty acids such as linoleic acid, oleic acid, stearic acid eichosanoic acids and palmitic acid, with more than 80% of the content being unsaturated fatty acids. Other trace compounds reported include Vitamin E (tocopherols), β-sitosterol, ad terpenes (e.g. myrcene and caryophyllene) and salicylates.
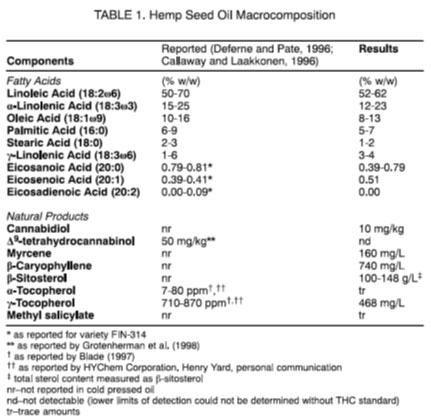
Given this information, hemp seed oil products should not contain significant amounts of cannabinoids. The presence of cannabinoids in hemp seed oil is considered to arise from either a contamination or adulteration, rather than be naturally occurring.
Pre-meeting public submissions
Three (3) public submissions were received for cannabis. Of these, 2 were opposed to the proposed amendments, and one proposed an additional amendment. The main points were:
- Concern that the level of caution concerning cannabis and its constituents is far higher than warranted given the suggested therapeutic benefit;
- The US legislation is being approved to allow personal use in addition to medical use;
- Multiple studies show that CBD is safe, and it is not appropriate to set any limit in hemp seed oil;
- Concern that there is ambiguity in the current Schedule 9 entries for cannabis and tetrahydrocannabinols (THC) and that the scheduling of low-THC hemp and hemp seed oils should also be exempt (less than 50 mg/kg);
- Concern that products not intended for therapeutic use (e.g. cosmetics and dog food) will be captured in the Schedule 8 and Schedule 9 entries and whether this is appropriate;
- One submission proposed that Schedule 9 entries for cannabis and tetrahydrocannabinols both be amended to include the following entry: "d) in products for the purpose other than internal human consumption use containing 50 mg/kg or less of tetrahydrocannabinols".
Summary of Joint ACCS-ACMS advice to the delegates
The committee advised that the Schedule 9 and Schedule 8 entries for cannabis and Schedule 9 entry for tetrahydrocannabinols be amended as follows:
Schedule 9 - Proposed Amended Entry
CANNABIS (including seeds, extracts, resins, and the plant and any part of the plant when packed or prepared), except:
- when separately specified in these Schedules; or
- processed hemp fibre containing 0.1 per cent or less of tetrahydrocannabinols, and hemp fibre products manufactured from such fibre; or
- in hemp seed oil for purposes other than internal human use containing 20 mg/kg or less of tetrahydrocannabinols and 50 mg/kg or less of total cannabinoids when labelled with either of the following warning statements:
- Not for internal use; or
- Not to be taken.
- in products for the purposes other than internal human use containing 20 mg/kg or less of tetrahydrocannabinols and 50 mg/kg or less of total cannabinoids.
Schedule 8 - Proposed Amended EntryCANNABIS (including seeds, extracts, resins and the plant, and any part of the plant) when prepared or packed for human therapeutic use, when:
- cultivated or produced, or in products manufactured[10], in accordance with the Narcotic Drugs Act 1967; and/or
- for use in products manufactured in accordance with the Narcotic Drugs Act 1967; and/or
- imported as therapeutic goods, or for use in therapeutic goods, for supply, in accordance with the Therapeutic Goods Act 1989; and/or
- in therapeutic goods supplied in accordance with the Therapeutic Goods Act 1989,
- except when:
- it is a product to which item 4, 8, 10, 11 or 12 of Schedule 5A to the Therapeutic Goods Regulations 1990 applies; or
- separately specified in Schedule 4; or
- separately specified in the NABIXIMOLS entry in this Schedule; or
- in hemp seed oil for purposes other than internal human therapeutic use containing 20 mg/kg or less of tetrahydrocannabinols and 50 mg/kg or less of total cannabinoids when labelled with either of the following warning statements
- Not for internal use; or
- Not to be taken.
Schedule 9 - Proposed Amended Entry
TETRAHYDROCANNABINOLS and their alkyl homologues, except:
- when included in Schedule 4 or Schedule 8; or
- processed hemp fibre containing 0.1 per cent or less of tetrahydrocannabinols, and hemp fibre products manufactured from such fibre; or
- in hemp seed oil, containing
5020 mg/kg or less of tetrahydrocannabinols and 50 mg/kg or less of total cannabinoids when labelled with either of the following warning statements:- Not for internal use; or
- Not to be taken; or
- in products for purposes other than internal human use containing
5020 mg/kg or less of tetrahydrocannabinols and 50 mg/kg or less of total cannabinoids.
Schedule 8 - Proposed Amended Entry
# TETRAHYDROCANNABINOLS when extracted from cannabis for human therapeutic use, when:
- included in products manufactured in accordance with the Narcotic Drugs Act 1967; and/or
- imported as therapeutic goods, or for use in therapeutic goods, for supply, in accordance with the Therapeutic Goods Act 1989; and/or
- in therapeutic goods supplied in accordance with the Therapeutic Goods Act 1989,
- except when:
- it is in a product to which item 4, 8, 10, 11 or 12 of Schedule 5A to the Therapeutic Goods Regulations 1990 applies; or
- in hemp seed oil, containing
5020 mg/kg or less of tetrahydrocannabinols and 50 mg/kg or less of total cannabinoids when labelled with either of the following warning statements:- Not for internal use; or
- Not to be taken; or
- in products for purposes other than for internal human use containing 50 mg/kg or less of tetrahydrocannabinols; or
- separately specified in the NABIXIMOLS entry in this Schedule.
The committee recommended that the schedule entry for cannabidiol be amended in Schedule 4 of the Poisons Standard to include a consistent hemp seed oil exemption:
Schedule 4 - Proposed Amended Entry
CANNABIDIOL in preparations for therapeutic use containing 2 per cent or less of other cannabinoids found in cannabis, except when:
- in hemp seed oil for purposes other than internal human use containing 20 mg/kg or less of tetrahydrocannabinols and 50 mg/kg or less of total cannabinoids when labelled with either of the following warning statements:
- Not for internal use; or
- Not to be taken.
- in products for the purposes other than internal human use containing 20 mg/kg or less of tetrahydrocannabinols and 50 mg/kg or less of total cannabinoids.
The committee recommended an implementation date of 1 June 2017.
The matters under subsection 52E (1) of the Therapeutic Goods Act 1989 considered relevant by the Committee included: a) the risks and benefits of the use of a substance; b) the purposes for which a substance is to be used and the extent of use of a substance; c) the toxicity of a substance; d) the dosage, formulation, labelling, packaging and presentation of a substance; e) the potential for abuse of a substance; f) any other matters that the Secretary considers necessary to protect the public health.
The reasons for the advice comprised the following:
- There is low risk associated with the concentration of cannabinoids permitted under the exceptions.
- Hemp seed oil contains fatty acids considered useful as skin conditioners and topical use is low risk, particularly if the level of psychoactive cannabinoid is minimal.
- Low THC hemp seed oil has been used in cosmetic and pet food products. Limiting human use to 'external only' mitigates against risk of internal consumption of cannabinoids, particularly tetrahydrocannabinols and cannabidiol.
- The risk of toxicity is minimal in the concentrations permitted under the exceptions Most of the toxicity associated with cannabis is due to the tetrahydrocannabinols (THCs) content.
- The toxicity will be low if the THC content is low. International jurisdictions have cut-off limits lower than 50 mg/kg; some jurisdictions have as low as 10 mg/kg.
- Label warning statements 'not for internal use' or 'not to be taken' would apply and make it clear that the products are not for human internal use.
- Including specific instructions about "Not for internal use" and/or "Not to be taken" makes it clear that oral formulations are not exempted from scheduling.
- There does not appear to be any evidence of misuse or abuse of the products that currently contain low concentrations of tetrahydrocannabinols/cannabinoids.
- Limiting the tetrahydrocannabinols content for exemption from scheduling reduces the risk of abuse and diversion.
- The amendments to the schedule entries would provide clarity and avoid any ambiguity about the products intended to be captured.
- There is merit in having consistent exemptions across all cannabis and tetrahydrocannabinols entries in Schedules 8 and 9 and the Schedule 4 entry for cannabidiol.
Delegate's considerations
The delegates considered the following in regards to this proposal:
- Scheduling proposal
- Joint ACCS-ACMS advice
- Public submissions received
- Section 52E of the Therapeutic Goods Act 1989
- Scheduling Policy Framework (SPF 2015)
- Other relevant information.
Delegates' interim decision
The delegates' interim decision is that:
- the Schedule 8 and 9 entries for cannabis and tetrahydrocannabinols be amended to remove the text 'internal' relating to human use
- the 'hemp seed oil' clauses in the Schedule 9 entries for cannabis and tetrahydrocannabinols be amended to:
- limit total cannabinoid content to 50 mg/kg including a new limit for tetrahydrocannabinols of 20 mg/kg
- restrict use in humans
- include labelling with either of the following warning statements 'not for internal use' or 'not to be taken'
- the Schedule 8 cannabis and tetrahydrocannabinols entries be amended by deleting the exemptions for 'hemp seed oil' and 'products'
- the Schedule 9 entry for tetrahydrocannabinols be amended by deleting the exemption for 'products'
- the Schedule 4 entry for cannabidiol be amended to include clarification in relation to total content of other cannabinoids.
The proposed wording for the Schedule 8 and Schedule 9 entries for cannabis and tetrahydrocannabinols and the Schedule 4 entry for cannabidiol are as follows:
Schedule 9 - Amend Entry
# CANNABIS (including seeds, extracts, resins, and the plant and any part of the plant when packed or prepared), except:
- when separately specified in these Schedules; or
- processed hemp fibre containing 0.1 per cent or less of tetrahydrocannabinols, and hemp fibre products manufactured from such fibre; or
- in hemp seed oil for purposes other than human use containing 50 mg/kg or less of total cannabinoids, including 20 mg/kg or less of tetrahydrocannabinols, when labelled with either of the following warning statements:
- Not for internal use; or
- Not to be taken.
Schedule 8 - Amend Entry
# CANNABIS (including seeds, extracts, resins and the plant, and any part of the plant) when prepared or packed for human therapeutic use, when:
- cultivated or produced, or in products manufactured[1], in accordance with the Narcotic Drugs Act 1967 and/or
- for use in products manufactured in accordance with the Narcotic Drugs Act 1967 and/or
- imported as therapeutic goods, or for use in therapeutic goods, for supply, in accordance with the Therapeutic Goods Act 1989; and/or
- in therapeutic goods supplied in accordance with the Therapeutic Goods Act 1989,
- except when:
- it is a product to which item 4, 8, 10, 11 or 12 of Schedule 5A to the Therapeutic Goods Regulations 1990 applies; or
- separately specified in Schedule 4; or
- separately specified in the NABIXIMOLS entry in this Schedule.
Schedule 9 - Amend Entry
# TETRAHYDROCANNABINOLS and their alkyl homologues, except:
- when included in Schedule 4 or Schedule 8; or
- processed hemp fibre containing 0.1 per cent or less of tetrahydrocannabinols, and hemp fibre products manufactured from such fibre; or
- in hemp seed oil for purposes other than human use containing 50 mg/kg or less of total cannabinoids, including 20 mg/kg or less of tetrahydrocannabinols when labelled with either of the following warning statements:
- Not for internal use; or
- Not to be taken.
Schedule 8 - Amend Entry
# TETRAHYDROCANNABINOLS when extracted from cannabis for human therapeutic use, when:
- included in products manufactured in accordance with the Narcotic Drugs Act 1967; and/or
- imported as therapeutic goods, or for use in therapeutic goods, for supply, in accordance with the Therapeutic Goods Act 1989; and/or
- in therapeutic goods supplied in accordance with the Therapeutic Goods Act 1989,
- except when:
- it is in a product to which item 4, 8, 10, 11 or 12 of Schedule 5A to the Therapeutic Goods Regulations 1990 applies; or
- separately specified in the NABIXIMOLS entry in this Schedule.
Schedule 4 - Amend Entry
CANNABIDIOL in preparations for therapeutic use containing 2 per cent or less of total other cannabinoids found in cannabis.
Appendix D, Item 1 - Current entries
CANNABIS for human use.
TETRAHYDROCANNABINOLS for human use.
Appendix K - Current entries
CANNABIS
TETRAHYDROCANNABINOLS
The proposed implementation date is 1 June 2017.
The delegates considered the relevant matters under section 52E (1) of the Therapeutic Goods Act 1989: a) the risks and benefits of the use of a substance; b) the purposes for which a substance is to be used and the extent of use of a substance; c) the toxicity of a substance; d) the dosage, formulation, labelling, packaging and presentation of a substance; e) the potential for abuse of a substance; f) any other matters that the Secretary considers necessary to protect the public health.
The reasons for the interim decision are the following:
- The delegates acknowledge the committee's advice.
- There is low risk associated with the concentration of cannabinoids permitted under the exceptions. The toxicity will be low if the THC content is low. International jurisdictions have cut off limits lower than 50 mg/kg; some jurisdictions have as low as 10 mg/kg.
- Low THC hemp seed oil has been used in cosmetic and pet food products. Limiting human use to 'external only' mitigates against risk of internal consumption of cannabinoids, particularly tetrahydrocannabinols and cannabidiol. Hemp seed oil contains fatty acids considered useful as skin conditioners and topical use is a low risk, particularly if the level of psychoactive cannabinoid is minimal.
- Label warning statements 'not for internal use' or 'not to be taken' would apply and make it clear the products are not for human internal use. Including specific instructions about "Not for internal use" and/or "Not to be taken" makes it clear that oral formulations are not exempted from scheduling.
- There does not appear to be any evidence of misuse or abuse of the products that currently contain low concentrations of tetrahydrocannabinols/cannabinoids. Limiting the tetrahydrocannabinols content for exemption from scheduling reduces the risk of abuse and diversion.
- The amendments to the schedule entries would provide clarity and avoid any ambiguity about the products intended to be captured. There is merit in having consistent exemptions across all cannabis and tetrahydrocannabinols entries in Schedules 8 and 9 and the Schedule 4 entry for cannabidiol, in particular the limits for total cannabinoids and tetrahydrocannabinols.
- The product exemption applying to products for purposes other than internal human use containing 50 mg/kg or less of tetrahydrocannabinols is being omitted as this is inconsistent with the operation of the Narcotic Drugs Act 1967 (the ND Act) and may be breaching Australia's obligations under the Single Convention on Narcotic Drugs 1961(the Single Convention). Any manufacture of drugs would be regulated under ND Act, and would require the manufacturer to be holding a manufacture licence and a permit. In view of the recent amendments to the ND Act, the Secretary must refuse to grant a manufacture licence involving cannabis, unless satisfied on reasonable grounds that at least one of the circumstances set out in subsection 11K(2) of the ND Act is met. Thus the end use of the manufactured cannabis under the ND Act is limited for a person to be granted a manufacture licence, irrespective of the concentration of cannabis in the end product to be supplied. Similarly, the cultivation and production of cannabis or cannabis resins are regulated under the ND Act.
- Any person who manufactures, cultivates cannabis plants or produces cannabis or cannabis resins without a licence may be committing an offence under the Criminal Code Act. Any importation of drugs would be regulated under the Customs (Prohibited Imports) Regulations 1956.
- The Single Convention does not apply to the cultivation of cannabis plants exclusively for industrial purposes (fibre and seed) or horticultural purposes. However, it applies to the cultivation of cannabis plants for the production of cannabis or cannabis resins, and requires amongst others that the manufacture of drugs be under licence, subject to exemptions, and that trade in and distribution of drugs be under licence, subject to exemptions.
- The cannabidiol Schedule 4 entry covers only therapeutic use. Therefore non-therapeutic use falls under other Schedule entries for cannabis.
- The cannabidiol entry amendment is to clarify that the cannabidiol must contain at least 98 per cent cannabidiol relative to the total amount of other cannabinoids in the cannabidiol.
- Amending the Schedule 9 entries for cannabis and tetrahydrocannabinols to introduce specific limits for total cannabinoids including tetrahydrocannabinols.
- NICNAS have advised that there are no cannabinoids approved as ingredients in cosmetics (i.e. external human use). Therefore there is no requirement for an exemption, as no approved products exist. This would lead to removal of the exemptions for hemp seed oil from the tetrahydrocannabinols and cannabis Schedule 8 entries and removal of the exemptions for products from the tetrahydrocannabinols and cannabis Schedule 9 and Schedule 8 entries.
- Food is not considered as part of this decision.
- Earliest possible implementation date.
Footnotes
- The November 2016 Poisons Standard contains an error, referring to 'cannabinols' instead of 'cannabinoids' under the Schedule 9 cannabis entry at item c. This will be corrected in the February 2017 update to be consistent with the August 2016 decision, pending the outcome of the advice of the committees.
- "Cultivation", "production" and "manufacture" have the same meaning as in the Narcotic Drugs Act 1967
- "Cultivation", "production" and "manufacture" have the same meaning as in the Narcotic Drugs Act 1967
- P1042 - Low THC Hemp Seeds as Food and Leizer, C. et al., The Composition of Hemp Seed Oil and Its Potential as an Important Source of Nutrition (pdf, 145kb), J. Nutraceuticals, Functional & Medical Foods 2000 2(4) 35 - 53



