Advisory Committee on Medicines Scheduling (ACMS #22)
On this page: Referred scheduling proposal | Scheduling application |Current scheduling status | Scheduling history | Australian regulatory information | International regulations | Substance summary | Pre-meeting public submissions | Summary of ACMS advice to the delegates | Delegate's considerations | Delegate's interim decision
Referred scheduling proposal
A delegate from the Therapeutic Goods Administration (TGA) has referred the substance hyaluronic acid for consideration to amend the Schedule 4 entry to include the subclause 'for intra-articular injection' in the Standard for the Uniform Scheduling of Medicines and Poisons (SUSMP) – the Poisons Standard.
Scheduling application
This was a delegate initiated application. The delegate's proposed amendments to the Poisons Standard are:
Schedule 4 – Amend Entry
HYALURONIC ACID AND ITS POLYMERS in preparations for injection or implantation:
- for tissue augmentation;
- for cosmetic use;
or - for the treatment of animals; or
- for intra-articular injection.
The delegate's reasons for the request are:
Appendix A
MEDICAL DEVICES classified as Class III by the classification rules set out in Schedule 2 to the Therapeutic Goods (Medical Devices) Regulation 2002 except:
- injectable tissue reconstructive, augmentation and restoration materials, including collagen;
- medical devices which include anticoagulants;
- artificial tears;
- urinary catheters; or
- intra-articular fluids.
"(a) the device will not compromise the clinical condition or safety of a patient, or the safety and health of the user or any other person, when the device is used on a patient under the conditions and for the purposes for which the device was intended and, if applicable, by a user with appropriate technical knowledge, experience, education or training;"
XXXX is intended to be used for intra-articular injection for symptomatic treatment of knee osteoarthritis.
Functional description:
XXXX is presented in a glass syringe with a rigid tip cap and plunger stopper. The syringe is assembled with a luer lock adapter, finger grip and plunger rod. XXXX is injected into the synovial joint by an authorised person experienced in intra-articular injections following an aseptic technique using an 18 to 22 gauge needle (not supplied). XXXX provides mechanical joint lubrication to the injected joint.
- The use of a product containing hyaluronic acid intended to be used for intra-articular injection for symptomatic treatment of knee osteoarthritis is not specifically noted in the Schedule 4 entry for hyaluronic acid.
- Based on the current schedule entry for hyaluronic acid in the Poisons Standard and on its scheduling history (particularly noting the June 2003 National Drugs and Poisons Schedule Committee (NDPSC) meeting – see Scheduling History below), hyaluronic acid in a registered product appears to be exempt from scheduling and does not require a prescription.
- According to the Australian Register of Therapeutic Goods (ARTG), the product is a medical device. According to the Appendix A entry for medical devices, medical devices are generally exempt from scheduling. However, this exemption does not extend to the product as it is intended to be used for intra-articular injection for symptomatic treatment of knee osteoarthritis. (See subclause) of the Appendix entry below:
- Sponsors of devices are obliged to ensure that the devices they supply in Australia meet the Australian Essential Principles. Essential Principle 1 of the Medical Devices Regulations 2002 states that:
- A medical device is to be designed and produced in a way that ensures that:
- Schedule 1, Part 2, subsection 13 states that the information supplied with the device (instructions for use) must identify how to use the device safely including having regard to the training and knowledge of potential users of the device. This means that the device itself, or the supplied instructions for use must clearly state that the device is intended to only be used by an authorised physician.
- The intent of the Medical Devices Regulations 2002 - external site is that the kinds of devices that should only be used by certain health care professionals are only marketed and supplied to these people. XXXX is such a device, as (according its ARTG entry):
- Prescriptions are not used for these types of devices, as the product is not supplied directly to the patient but rather to a health care professional who would then directly administer it to the patient. This is unlike a medicine supplied through a prescription, which the patient would obtain themselves using a prescription and then administer it themselves.
- The sponsor of this device (or their agent) should not be marketing this device to a pharmacy that would then supply to the general public.
- The intention of Schedule 4, part c) 'for the treatment of animals' was to allow for the use of hyaluronic acid via intra-articular injection in animals (see June 2003 NDPSC meeting – see Scheduling History below). The Schedule 4 entry currently excludes this use in humans.
Current scheduling status
Hyaluronic acid and its polymers are in Schedule 4 of the current Poisons Standard as follows:
Schedule 4
HYALURONIC ACID AND ITS POLYMERS in preparations for injection or implantation:
- for tissue augmentation;
- for cosmetic use; or
- for the treatment of animals.
Scheduling history
The relevant scheduling history of hyaluronic acid is as follows:
May 1986 National Drugs and Poisons Schedule Committee (NDPSC)
In May 1986, the NDPSC agreed to include hyaluronic acid in preparations for injection in Schedule 4, on the grounds that the product containing hyaluronic acid was used to treat a serious condition requiring veterinary intervention.
May 2000 National Drugs and Poisons Schedule Committee (NDPSC)
In May 2000, the committee were made aware that certain products that contained hyaluronic acid may have been overlooked at the time of registration and were classified as a device.
February 2001 National Drugs and Poisons Schedule Committee (NDPSC)
In February 2001, the NDPSC considered a background paper on hyaluronic acid use in devices. The committee had become aware that many products classified as devices for registration purposes, contained scheduled substances. The committee also considered the Trans-Tasman Harmonisation Working Party (TTHWP) recommendation that New Zealand adopt the existing Schedule 4 entry for hyaluronic acid. Additionally, the committee considered a request that the Schedule 4 entry for hyaluronic acid in the Standard for the Uniform Scheduling of Drugs and Poisons (SUSDP) be reviewed, to exempt certain products, containing hyaluronic acid manufactured by bacterial fermentation.
This was deferred until the next meeting to allow assessment of the appropriateness and feasibility of scheduling substances that are either included in products, or are products, regulated as medical devices.
May 2001 National Drugs and Poisons Schedule Committee (NDPSC)
In May 2001, the NDPSC agreed that for clarity, the entry for hyaluronic acid in the SUSDP be amended to include its polymers. However, the committee did not agree that injectable products containing hyaluronic acid should not be exempt from scheduling but that these should be subject to the requirements of Schedule 4. The committee agreed to include the entry 'HYALURONIC ACID and its polymers, in preparations for injection.' in Schedule 4 of the SUSDP, and an entry for intraocular viscoelastic products in Appendix A.
August 2001 National Drugs and Poisons Schedule Committee (NDPSC)
In August 2001, the NDPSC considered hyaluronic acid when in intraocular viscoelastic products and confirmed that the Appendix A entry for viscoelastic products remained appropriate.
February 2002 National Drugs and Poisons Schedule Committee (NDPSC)
In February 2002, NDPSC considered a proposal to amend the existing entry for hyaluronic acid to read, 'HYALURONIC ACID AND ITS POLYMERS in preparations for injection.' Members agreed that the proposed amendment to change 'hyaluronic acid and its polymers' to upper case letters would ensure a consistent interpretation of the entry across the jurisdictions. The committee decided that such an amendment would be appropriate given that there is no regulatory impact expected as a result of the amendment.
February 2003 National Drugs and Poisons Schedule Committee (NDPSC)
In February 2003 the NDPSC amended the Schedule 4 entry for hyaluronic acid to include preparations for implantation. The committee agreed to clarify the intent of Schedule 4 entries for substances used in tissue augmentation or cosmetic use (including collagen, hyaluronic acid and polylactic acid), to encompass preparations for injection or implantation. The Schedule 4 hyaluronic acid amendment was made as follows:
HYALURONIC ACID in preparations for injection or implantation:
- for tissue augmentation; or
- for cosmetic use
June 2003 National Drugs and Poisons Schedule Committee (NDPSC)
In June 2003 the NDPSC agreed that the to the Schedule 4 entry amendment for hyaluronic acid inadvertently excluded 4 veterinary products containing sodium hyaluronate for the treatment of non-infectious joint diseases (synovitis) of horses. The committee recognised that the unintended regulatory impact on the Schedule 4 veterinary products and noted that the products required veterinary intervention so should remain in Schedule 4. The committee agreed that the Schedule 4 entry for hyaluronic acid should be amended to include 'c) for the treatment of animals'. The Schedule 4 hyaluronic acid amendment was made as follows:
HYALURONIC ACID in preparations for injection or implantation:
- for tissue augmentation; or
- for cosmetic use
- for the treatment of animals.
Australian regulatory information
According to the TGA Ingredient Database - external site, hyaluronic acid is permitted to be used as an:
- Excipient ingredient in Biologicals, Export Only, Listed Medicines, Over the Counter and Prescription Medicines; and
- Active ingredient in Biologicals, Export Only and Prescription Medicines.
Hyaluronic acid is not currently used in proprietary ingredient (PI) formulation.
Hyaluronic acid is listed in the Therapeutic Goods (Permissible Ingredients) Determination No. 4 of 2017 - external site as an excipient in topical medicines for dermal applications.
In the last 30 years, there has been one adverse event reported in the Database of Adverse Event Notifications – Medicines - external site with hyaluronic acid listed as a single suspected medicine. The reported adverse effect was hypoaesthesia.
There are 2 products on the ARTG containing hyaluronic acid:
- APVMA has 8 products registered for use in horses containing sodium hyaluronate. No adverse events were recorded from January 1995 to December 2013.
International regulations
Canada
In Canada, hyaluronic acid for intra articular use is regulated as class III or class IV medical devices and sold as pre-filled single use syringes.
Hyaluronic acid is also permitted for intra-articular use in horses.
United States of America
In the USA, hyaluronic acid is permitted for use in intra articular injections, and approved dermal fillers.
Hyaluronic acid is also permitted for intra articular use in horses, subject to conditions of use related to the dosage and treatment regime.
European Union (EU)
In the EU, there are no apparent restrictions on the use of hyaluronic acid.
New Zealand
In NZ, hyaluronic acid is considered prescription only for injections or implants for tissue augmentation or cosmetic use, and for general sale for all other uses.
Substance summary
Hyaluronic acid in its natural form is a linear unbranched (no isomerisation) biological polymer found in all animals, some plants and some bacteria (particularly gram positive). It is a glycosaminoglycan. The hyaluronic acid polymer is composed of repeating units of D-glucuronic acid and N-acetyl-D-glucosamine disaccharide units.
| Property | Hyaluronic acid |
|---|---|
| CAS number | 9004-61-9 |
| IUPAC and/or common and/or other names | (2S,4S,5R,6S)-6-[(2S,3R,5S,6R)-3-acetamido-2-[(3S,4R,5R,6R)-6-[(3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid (IUPAC); Hyaluronan; Hyvisc; Luronit; Vitrax, Amp |
| Chemical structure | 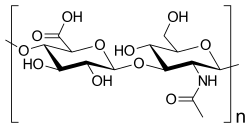 |
| Molecular formula | Polymer: (C14H21NO11)n Monomer: C28H44N2O23, n = 2 |
| Molecular weight | 776.7 g/mol (monomer) |
N-Acetyl-D-glucosamine is a component of connective tissue, skin, vitreous humour, umbilical cord, synovial fluid and the capsule of certain microorganisms contributing to adhesion, elasticity, and viscosity of extracellular substances. It is also found as small saccharides in the tissue due to enzymatic digestion and chemical breakdown.
Hyaluronan is a commonly term for salts of hyaluronic acid (usually Na, but exists in the tissues as various salts and complexes) but is also used interchangeably with the acid. Hyaluronic acid and its cross-linked derivatives are also used in tissue augmentation and visco-supplementation. Although the cross-linked forms have better residence time, resist enzymatic degradation and could have superior rheological properties in some applications. In literature, the term hylan is used to describe hyaluronic acids cross-linked with highly reactive chemicals (e.g. divinyl sulfone, imides or dialdehyde).
Pharmacology and Biochemistry
Hyaluronic acid is similar to a substance that occurs naturally in the joints. It may act as a lubricant and shock absorber in the joint, helping the joint to move smoothly.
Drug indication
Hyaluronic acid is used to treat knee pain in patients with joint inflammation (osteoarthritis). It is usually used in patients who have not responded to other treatments such as paracetamol, exercise, or physical therapy. Hyaluronic acid may also be used in plastic surgery to reduce wrinkles on the face or as a filler in other parts of the body. It may be used in ophthalmology to assist in the extraction of cataracts, the implantation of intraocular lenses, corneal transplants, glaucoma filtration, retinal attachment and in the treatment of dry eyes and is also used to coat the bladder lining in treating interstitial cystitis.
Drug warnings
Hyaluronic acid is considered to be non-immunogenic and is frequently used for the correction of facial lines. It is believed that hyaluronic acid injection fillers are safe and have no occurrence of serious adverse reactions or allergic reactions. However, publications have documented the rate of intermittent swelling and severe granulomatous allergic reactions that evolved into abscesses. Literature describes a clinical case of a 54-year-old patient.[1] After injection of hyaluronic acid in the treatment of nasolabial folds, palpable painful erythematous nodules evolved into abscesses several months after injection. Surgical treatment and correction of the lesions after the hyaluronic acid injection of the nasolabial folds and histological findings of the erythematous nodules were described. Histological and clinical examination documented intermittent swelling and severe granulomatous allergic reactions that may render the use of hyaluronic acid unacceptable. The reference recommends that patients should be informed of the potential complications when treating facial lines with hyaluronic acid gel.
Non-animal hyaluronic acid gel was developed for soft tissue augmentation and volume expansion and has been reported to offer several advantages in comparison to other augmentation materials. There are rare reports of adverse events believed to be secondary to trace amounts of proteins in the hyaluronic acid raw material. Data from an estimated 144,000 patients treated in 1999 indicated the major reaction to injectable hyaluronic acid was localised hypersensitivity reactions, occurring in approximately 1 of every 1400 patients treated. In 1999, there was an adverse event reported for 1 of every 650 patients (0.15%) treated. These were temporary events that included redness, swelling, localised granulomatous reactions, bacterial infection, as well as acneiform and cystic lesions. In 2000, there was an estimated 262,000 patients treated with hyaluronic acid gel. The total number of adverse events was 144, corresponding to one adverse event for every 1800 patients (0.06%) treated. The major adverse event was again hypersensitivity, occurring in 1 of every 5000 patients treated. According to the reported worldwide adverse events data, hypersensitivity to non-animal hyaluronic acid gel is the major adverse event and is most likely secondary to impurities of bacterial fermentation. According to data from 2000, the incidence of hypersensitivity appears to be declining after the introduction of a more purified hyaluronic acid raw material.[2]
Absorption, distribution and excretion
Hyaluronic acid is absorbed and diffuses slowly out of the injection site. It is eliminated via the canal of Schlemm and is degraded by hyaluronidase enzymes.
Mechanism of action
Hyaluronic acid functions as a tissue lubricant and is thought to play an important role in modulating the interactions between adjacent tissues. Hyaluronic acid is a polysaccharide which is distributed widely in the extracellular matrix of connective tissue. It forms a viscoelastic solution in water which makes it suitable for aqueous and vitreous humor in ophthalmic surgery. Mechanical protection for tissues (iris, retina) and cell layers (corneal, endothelium, and epithelium) are provided by the high viscosity of the solution. Elasticity of the solution assists in absorbing mechanical stress and providing a protective buffer for tissues. This viscoelasticity enables maintenance of a deep chamber during surgical manipulation since the solution does not flow out of the open anterior chamber. In facilitating wound healing, it is thought that it acts as a protective transport vehicle, taking peptide growth factors and other structural proteins to a site of action. It is then enzymatically degraded and active proteins are released to promote tissue repair. Hyaluronic acid is being used intra-articularly to treat osteoarthritis. Cell receptors that have been identified for hyaluronic acid fall into three main groups: CD44, Receptor for Hyaluronan-mediated motility (RHAMM) and intracellular adhesion molecule-1 (ICAM-1). CD44 mediates cell interaction with hyaluronic acid and the binding of the two functions as an important part in various physiologic events, such as cell aggregation, migration, proliferation and activation; cell-cell and cell-substrate adhesion; endocytosis of hyaluronic acid, which leads to hyaluronic acid catabolism in macrophages; and assembly of petircellular matrices from hyaluronic acid and proteoglycan. CD44 has two important roles in skin, regulation of keratinocyte proliferation in response to extracellular stimuli and the maintenance of local hyaluronic acid homeostasis. ICAM-1 is known mainly as a metabolic cell surface receptor for hyaluronic acid, and this protein may be responsible mainly for the clearance of hyaluronic acid from lymph and blood plasma, which accounts for perhaps most of its whole-body turnover. Ligand binding of this receptor, thus, triggers a highly coordinated cascade of events that includes the formation of an endocytotic vesicle, its fusion with primary lysosomes, enzymatic digestion to monosaccharides, active transmembrane transport of these sugars to cell sap, phosphorylation of GlcNAc and enzymatic deacetylation. ICAM-1 may also serve as a cell adhesion molecule, and the binding of hyaluronic acid to ICAM-1 may contribute to the control of ICAM-1-mediated inflammatory activation.
Pre-meeting public submissions
No submissions were received.
Summary of ACMS advice to the delegates
The committee recommended that the Schedule 4 entry for hyaluronic acid in the Poisons Standard be amended as follows:
Schedule 4 – Amend Entry
HYALURONIC ACID AND ITS POLYMERS in preparations for injection or implantation:
for tissue augmentation;for cosmetic use; orfor the treatment of animals.
The committee also recommends an implementation date of 1 June 2018 as this is the earliest practicable implementation date.
Members agreed that the relevant matters under Section 52E(1) of the Therapeutic Goods Act 1989 included: (a) risks and benefits of the use of a substance; (b) the purpose for which a substance is to be used and the and extent of use; (c) the toxicity of a substance; (d) the dosage, formulation, labelling, packaging and presentation of a substance; and (f) any other matters that the Secretary considers necessary to protect public health.
The reasons for the advice included:
- the risks and benefits of the use of a substance:
- Risks: Hyaluronic acid presents very little risk when used in accordance with TGA-approved Product Information.
- Benefits: The potential benefits are for people not responding to other standard treatments for knee osteoarthritis and also those awaiting joint replacements, etc. Additionally, it is a one-off treatment lasting for 6-9 months rather than having to take increased quantities of pain medication daily. The associated risks of this are especially so in the older population.
- the purposes for which a substance is to be used and the extent of use of a substance:
- For the symptomatic treatment of knee osteoarthritis
- Potential for misuse
- The ailments or symptoms that hyaluronic acid is used for require medical intervention.
- the toxicity of a substance:
- Very low risk of allergy, toxicity or systemic adverse events in humans.
- the dosage, formulation, labelling, packaging and presentation of a substance:
- Packaged as an injectable.
- The use of hyaluronic acid requires a specialised medicine delivery device.
- the potential for abuse of a substance:
- Nil
- any other matters that the Secretary considers necessary to protect public health:
- Appropriate that hyaluronic acid be administered under a health practitioner’s supervision.
Delegate's considerations
The delegate considered the following in regards to this proposal:
- Scheduling proposal
- ACMS advice
- Section 52E of the Therapeutic Goods Act 1989
- Scheduling Policy Framework (SPF 2015)
Delegate's interim decision
The delegate’s interim decision is to amend the Schedule 4 entry for hyaluronic acid. The proposed Schedule entry is:
Schedule 4 – Amend Entry
HYALURONIC ACID AND ITS POLYMERS in preparations for injection or implantation.
The proposed implementation date is 1 June 2018. This is the earliest practicable implementation date.
The matters under subsection 52E (1) of the Therapeutic Goods Act 1989 considered relevant by the delegate included: (a) the risks and benefits of the use of the substance; (b) the purposes for which a substance is to be used and the extent of use of a substance; (c) the toxicity of the substance; (d) the dosage, formulation, labelling, packaging and presentation of a substance; and (f) any other matters that the Secretary considers necessary to protect public health.
The reasons for the recommendation comprised the following:
- the risks and benefits of the use of a substance:
- Risks: Hyaluronic acid presents very little risk when used in accordance with TGA-approved Product Information.
- Benefits: The potential benefits are for people not responding to other standard treatments for knee osteoarthritis and also those awaiting joint replacements, etc. Additionally, it is a one-off treatment lasting for 6-9 months rather than having to take increased quantities of pain medication daily. The associated risks of this are especially so in the older population.
- the purposes for which a substance is to be used and the extent of use of a substance:
- For the symptomatic treatment of knee osteoarthritis.
- Potential for misuse.
- The ailments or symptoms that hyaluronic acid is used for require medical intervention.
- Any use as of an injection or implant requires medical practitioner use.
- the toxicity of a substance:
- Very low risk of allergy, toxicity or systemic adverse events in humans.
- the dosage, formulation, labelling, packaging and presentation of a substance:
- Packaged as an injectable.
- The use of hyaluronic acid requires a specialised medicine delivery device.
- the potential for abuse of a substance:
- Nil
- any other matters that the Secretary considers necessary to protect public health
- Appropriate that hyaluronic acid be administered under a health practitioner’s supervision.

