We will have limited operations from 15:00 Wednesday 24 December 2025 (AEDT) until Friday 2 January 2026. Find out how to contact us during the holiday period.
This is a step-by-step guide for agents and sponsors who wish to apply for Priority review or, Provisional determination and / or Orphan drug designation of a prescription medicine.
If you are logging in as a sponsor, some fields that are visible to agents will not be visible to you. This is because we already hold certain information about you and you do not need to re-enter it.
Before you apply
To make an application you will need:
- a TGA client ID number
- access to the TGA Business Services portal
If you do not have a client ID number or access to the TGA Business Services portal, go to TGA Business Services: getting started with TGA and submit the online organisation details form.
Completing your application
- Log in to TGA Business Services.
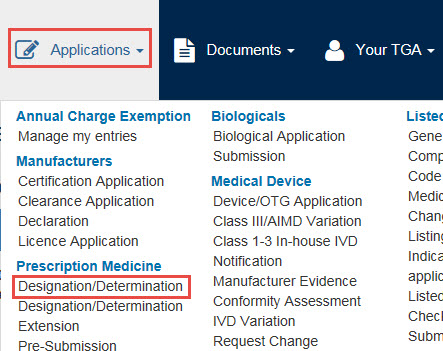
From the Applications dropdown menu, go to Prescription Medicine and select Designation/Determination.
An application window will open. This window offers four tabs:
- Applicant Details
- Application Scope
- Product Details
- Administration

On each tab there are a number of fields to fill in.
Fields marked with a red asterisk (*) are always mandatory. Fields marked with a grey asterisk (*) are mandatory in some circumstances.
Saving your draft application
The Applicant Details tab
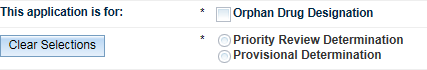
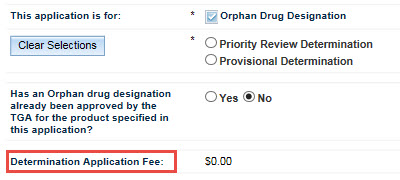
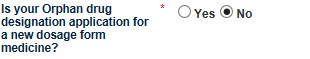
The Application Scope tab
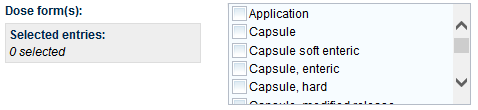
The Product Details tab
The Administration tab
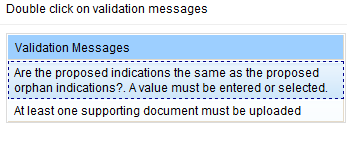
Validating your application
After you have saved the form, the Validate radio button will become active. You must validate your application before you can submit it.
Validating
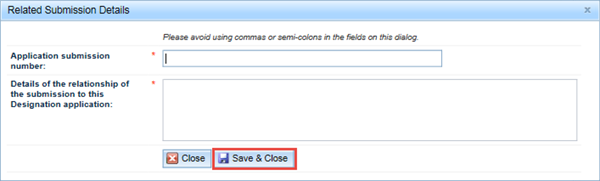
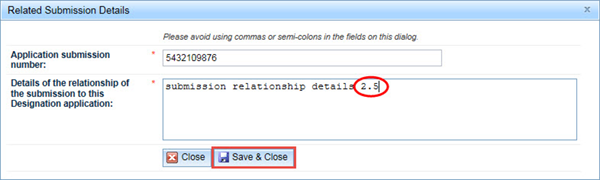
Submitting your application
When you are ready to submit your application, click on the Submit button at the bottom right of the browser window. This will open a Declaration pop-up window.
Declaration
Application assessment
For more information on how we will assess your application(s), see the Priority review determination, Provisional determination and Orphan drug designation step-by-step guides.
If you have read the user guide and still require assistance, please contact: AET.Application.Entry.Team@health.gov.au.
| Version | Description of change | Author | Effective date |
|---|---|---|---|
| V1.0 | Original publication | Prescription Medicines Authorisation Branch | July 2017 |
| V1.1 | Updates to include the provisional pathway and other minor edits | Prescription Medicines Authorisation Branch and Regulatory Guidance Team | March 2018 |