Consumers and health professionals are advised that Symbion has identified three additional batches affected by the issue described below and is adding them to the recall.
The additional products are:
- Pharmacy Choice Ibuprofen Children's Suspension (6 months – 12 years)
- Batch number: FA4514 (expiry date: 03/2017)
- Pharmacy Choice Children's Paracetamol 6 – 12 Years Concentrated.
- Batch number: IA50152 (expiry date: 01/2017)
- Batch number: IA45030 (expiry date: 10/2016)
All products were supplied in 200 mL bottles.
Information on what consumers and health professionals should do regarding these products can be found below.
Recall - risk of plastic fragments breaking from bottle and being ingested
6 July 2015
Consumers and health professionals are advised that Symbion, in consultation with the TGA, is recalling six batches of ibuprofen and paracetamol products after it was identified that the bottle grooves that secure the lids on these products could break off during opening.
The issue affects some batches of the following products supplied in 200 mL bottles:
- Chemmart Ibuprofen Children's Suspension (6 months - 12 years)
- Pharmacy Choice Ibuprofen Children's Suspension (6 months - 12 years)
- Chemmart Children's Paracetamol 6 - 12 Years Concentrated.
There is a risk that small pieces of plastic could be ingested if the issue is not identified by the user, potentially posing a choking hazard.
Ibuprofen and paracetamol are both used to relieve pain and fever, with ibuprofen also being used to reduce inflammation.
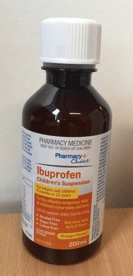

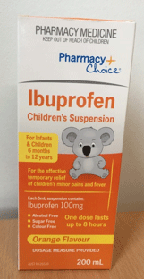

The bottles and outer packaging of the two affected ibuprofen products

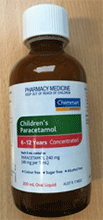
The outer packaging and bottle containing Chemmart Children's Paracetamol 6 - 12 Years Concentrated
The affected batch numbers are:
- Chemmart Ibuprofen Children's Suspension (6 months - 12 years):
- IA50030 (expiry date: 06/2017)
- IA45163 (expiry date: 05/2017)
- Chemmart Children's Paracetamol 6 - 12 Years Concentrated:
- IA45031 (expiry date: 10/2016)
- Pharmacy Choice Ibuprofen Children's Suspension (6 months - 12 years):
- IA50026 (expiry date: 06/2017)
- IA50044 (expiry date: 06/2017)
- IA45162 (expiry date: 05/2017).
Information for consumers
If you or someone you care for is using Chemmart or Pharmacy Choice branded Ibuprofen Children's Suspension, or Chemmart Children's Paracetamol 6 - 12 Years Concentrated, please be aware of this issue.
The issue has only been identified in the five batches of 200 mL Ibuprofen Children's Suspension listed above, branded as either Chemmart or Pharmacy Choice, and one batch of Chemmart Children's Paracetamol 6 - 12 Years Concentrated. No other batches are known to be affected.
If you have ibuprofen or paracetamol from the potentially affected batches, return the medicine to the place of purchase for refund or replacement. Alternatively, you can call Symbion's customer service line on 1300 430 611.
In the meantime do not use the affected batch.
Information for health professionals
If you are treating a patient who is using Chemmart or Pharmacy Choice branded Ibuprofen Children's Suspension, or Chemmart Children's Paracetamol 6 - 12 Years Concentrated, advise them, their parent or their carer of this issue.
If they are using ibuprofen or paracetamol from the potentially affected batches listed above, advise them to return the medicine to the place of purchase for refund or replacement.
Alternatively, they can call Symbion's customer service line on 1300 430 611.
In the meantime they should be advised not to use the affected batch.
Reporting problems
Consumers and health professionals are encouraged to report problems with medicines or vaccines. Your report will contribute to the TGA's monitoring of these products.
The TGA cannot give advice about an individual's medical condition. You are strongly encouraged to talk with a health professional if you are concerned about a possible adverse event associated with a medicine or vaccine.

