Consumers, health professionals and suppliers are advised that Ascent Pharmaceuticals, in consultation with the TGA, are recalling one batch of Febridol Paracetamol 500 mg 100 tablet bottles.
Febridol Paracetamol 500 mg contains paracetamol, which is used to treat aches, pains and feverish conditions.
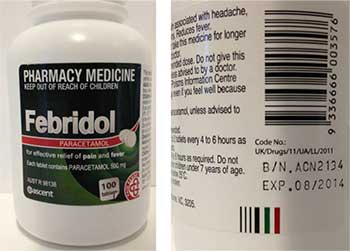 It has been found that one batch of Febridol Paracetamol 500 mg 100 tablet bottles (batch number ACN2134, expiry date August 2014), supplied in NSW and the ACT, may contain a foreign tablet that is peach (orange/pink) in colour and triangular in shape. It can be easily identified among Febridol Paracetamol 500 mg tablets, which are white and round.
It has been found that one batch of Febridol Paracetamol 500 mg 100 tablet bottles (batch number ACN2134, expiry date August 2014), supplied in NSW and the ACT, may contain a foreign tablet that is peach (orange/pink) in colour and triangular in shape. It can be easily identified among Febridol Paracetamol 500 mg tablets, which are white and round.
The manufacturer is undertaking an investigation to identify the peach-coloured tablet.
Please note that taking the peach-coloured tablet could be harmful.
No other batches of Febridol Paracetamol 500 mg, either in 100 tablet bottles or 100 tablet blister packs, are affected by this recall.
Information for consumers
If you have any Febridol Paracetamol 500 mg 100 tablet bottles, please check the batch number. If the batch number is ACN2134, with expiry date August 2014, do not take any medicine from that bottle. Return the bottle to the place of purchase for a refund, or call the Ascent Pharmaceuticals customer service line on 1800 678 302 to arrange the return of the product and a refund.
If you have any questions or concerns about this issue, please contact your pharmacist.
Information for health professionals and suppliers
Ascent Pharmaceuticals has written to pharmacies and wholesale suppliers, providing further information about this issue and the recall process.
Please inspect your stocks of Febridol Paracetamol 500 mg 100 tablet bottles to see if they are from the affected batch (batch number ACN2134, expiry date August 2014). If any such products are found, please quarantine those bottles and arrange to return them to Ascent Pharmaceuticals.
If you have any questions or concerns about this issue, please call the Ascent Pharmaceuticals customer service line on 1800 678 302.
Reporting problems
Consumers and health professionals are encouraged to report problems with medicines or vaccines. Your report will contribute to TGA's monitoring of these products.
The TGA cannot give advice about an individual's medical condition. You are strongly encouraged to talk with a health professional if you are concerned about a possible adverse event associated with a medicine or vaccine.

