Stryker, in consultation with the Therapeutic Goods Administration, is undertaking a Product Defection Correction to update the operating instructions of all LIFEPAK CR2 defibrillators and will be providing replacement parts where appropriate.
LIFEPAK CR2 defibrillators are automated external defibrillators (AEDs) used in cases of life-threatening irregular heartbeat (also known as cardiac arrhythmia or cardiac dysrhythmia) that leads to cardiac arrest. Defibrillators work by applying electricity to stop the arrhythmia, allowing the heart to re-establish an effective rhythm.

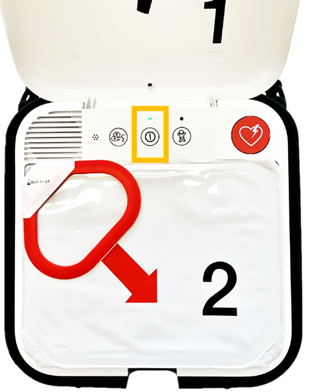
The LIFEPAK CR2 AED has a lid which usually automatically powers on and off the device as it is opened and closed, which is controlled by a lid magnet.
It has been identified that some LIFEPAK CR2 AEDs have a defect that may result in the lid magnet becoming dislodged. If the magnet becomes dislodged, there is a risk that the battery may become prematurely depleted and the device may not turn on automatically or work correctly. Instead, users must press the on/off button to operate the device.
To address this, Stryker recommends all LIKEPAK CR2 AEDs be checked carefully to ensure their lid magnets are securely in place. Where a lid magnet has been dislodged, Stryker will be providing replacement lids at no charge.

If you have a device with a dislodged lid magnet or that is not turning on when you open the lid, you can continue to use it by using its power button to turn it on and off and following the updated operating instructions.
Supplemental instructions are being sent to all owners of these devices, along with a letter about this issue (including details of which serial numbers have defective lid magnets and which do not, but should still be checked). This will help reduce the risk until replacement parts can be provided.
There have been two reported deaths in relation to this issue worldwide. There have been no reported injuries or deaths within Australia.
If you have a LIFEPAK CR2 AED and have any questions or concerns about this issue, contact your Stryker representative or Stryker's ProCare Service team by phone on 1800 667 558 or at ssptechservices@stryker.com.
Reporting problems
Consumers and health professionals are encouraged to report problems with medical devices. Your report will contribute to the TGA's monitoring of these products. For more information, see the TGA Incident Reporting and Investigation Scheme (IRIS).
The TGA cannot give advice about an individual's medical condition. You are strongly encouraged to talk with a health professional if you are concerned about a possible adverse event associated with a medical device.

