We will have limited operations from 15:00 Tuesday 24 December 2024 (AEDT) until Thursday 2 January 2025. Find out how to contact us during the holiday period.
Consumers and health professionals are advised that Clinect, in consultation with the TGA, has initiated a recall for product correction for Neria infusion sets with an expiry date on or before May 2019 (see below for a list of affected lots).
Please note that these products are not being removed from the market. Consumers will have the option of having the affected devices replaced free of charge, or continue using them in accordance with some additional instructions.
Neria infusion sets are used in conjunction with a pump to deliver subcutaneous infusion of medicines to help treat patients with a range of conditions including Parkinson's disease, primary immunodeficiency, chronic pain and thalassaemia.
There has been a slight increase in reports of cases where the needles of these infusion sets have broken during use, including one Australian adverse event report that may be related to this issue.
Devices manufactured after May 2014 with an expiry date of June 2019 or later are unaffected by this recall for product correction.
If the needle breaks during use, the delivery of medicine will be interrupted and the patient may experience a range of symptoms depending on therapy area and medication used. Additionally, if a broken needle remains in the body, it can lead to infection which, in rare cases, might require surgical removal.

Point where the needle can break
The affected lots are:
| Reference number (model) | Product description | Lot numbers |
|---|---|---|
| 78-060-2736 | Neria G27-6mm-60cm | 5017071, 5024517, 5033612 |
| 78-080-2738 | Neria G27-8mm-80cm | 227979, 227980, 5006113, 5006114, 5006115 |
| 78-080-2731 | Neria G27-10mm-80cm | 5028915, 5063419 |
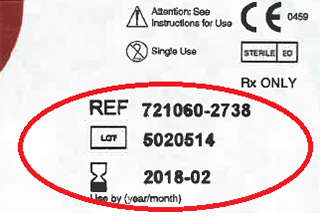
| 721060-2738 | Neria Multi 2 G27-8mm | 5020514, 5022947, 5033623, 5063787 |
Information for consumers
Clinect has written to Neria infusion set users to notify them of this issue and the actions they can take to address it.
If you or someone you care for uses a Neria infusion set, check the box or pouch labels to see if you have an affected device. These labels include a reference number relating to the model number, a lot number and an expiry date (marked with a red oval in the image).
If the reference and lot numbers match those listed in the table above, and if the expiry date is on or before May 2019, you should take one of the two following alternatives:
Replacement - Call Clinect on 1300 890 284 or email neria@clinect.com.au to arrange replacement of the infusion sets free of charge.
Continue use - You can continue to use the affected products if you carefully review the Instructions for Use included with the product, as well as follow the below additional instructions:
- Prior to use:
- Carefully remove the needle guard before inserting the infusion set. The needle guard should be removed without the use of any twisting or bending of the needle guard.
- Do not use the infusion set if the needle is bent or has been damaged.
- Do not bend the needle prior to insertion.
- Consult your health professional for the proper insertion site.
- After use:
- Carefully remove the infusion set after use to avoid twisting or bending of the needle.
- Ensure the complete needle is present on the used infusion set before discarding it.
- Contact your health professional if you suspect that a needle has broken off and remained under the skin.
- Monitor symptoms which could indicate that delivery of the medicine had failed.
Seek medical attention immediately if you are experiencing any symptoms.
If you have any questions or concerns about this issue, speak to a health professional. Alternatively, you can contact Clinect on 1300 890 284 (customer service) or 1800 899 005 (medical information), or email neria@clinect.com.au.
Information for health professionals
Clinect has written to consumers for whom it has contact details, health facilities and wholesalers alerting them to this issue.
If you are treating patients who use Neria infusion sets, please ensure they are aware of this issue and the actions they can take to address it (see the 'Information for consumers' section above for specific instructions).
If you have any questions or concerns about this issue telephone Clinect on 1300 890 284 (customer service) or 1800 899 005 (medical information), or email neria@clinect.com.au.
Reporting problems
Consumers and health professionals are encouraged to report problems with medical devices. Your report will contribute to the TGA's monitoring of these products. For more information see the TGA Incident Reporting and Investigation Scheme (IRIS).
The TGA cannot give advice about an individual's medical condition. You are strongly encouraged to talk with a health professional if you are concerned about a possible adverse event associated with a medical device.



