On this page: New applications | Draft applications
New applications
- Select the application type from the drop down under the 'Applications menu'.
- Under Listed medicine subheading the following options are displayed:
- General listed - containing a single listed medicine made under section 26A of the Act;
- Assessed listed - contains a single listed medicine made under section 26AB of the Act following assessment of efficacy;
- General Composite pack - allows for two or more medicine formulations to be included, forming a pack which is listed under section 26A of the Act;
- Assessed composite pack - allows for two or more medicine formulations to be included, forming a pack which is listed under section 26AB of the Act following assessment of efficacy;
- Medicine kit - enables two or more existing registered or listed medicines and an exempt good, to be grouped and sold together in a purpose built pack;
- Change Multiple Current Listings - allows for minor changes to be made to multiple general listed medicines.
Select the appropriate application type from the list. The information required for each application type will vary slightly.

Draft applications
Previously completed draft applications which have not been submitted can be accessed by selecting 'Work on drafts' from the My Work menu.
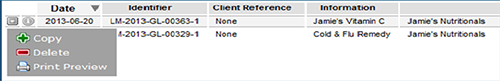
The Drafts window will open displaying a list of current draft applications. All types of listed medicines applications will be displayed in this window. Click on the draft application you wish to continue editing.
To access other functions such as 'copy', 'delete' or 'print preview', select the arrow to the left of the application.
NOTE: Draft applications are automatically deleted from the system if they have not been updated in the last twelve months. Once deleted, records are not retrievable.

