This is a step-by-step guide for agents and sponsors who wish to apply for Priority review or, Provisional determination and / or Orphan drug designation of a prescription medicine.
If you are logging in as a sponsor, some fields that are visible to agents will not be visible to you. This is because we already hold certain information about you and you do not need to re-enter it.
Before you apply
To make an application you will need:
- a TGA client ID number
- access to the TGA Business Services portal
If you do not have a client ID number or access to the TGA Business Services portal, go to TGA Business Services: getting started with TGA and submit the online organisation details form.
Completing your application
- Log in to TGA Business Services.
From the Applications dropdown menu, go to Prescription Medicine and select Designation/Determination.
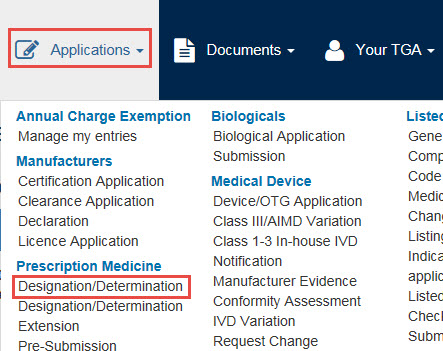
An application window will open. This window offers four tabs:
- Applicant Details
- Application Scope
- Product Details
- Administration

On each tab there are a number of fields to fill in.
Fields marked with a red asterisk (*) are always mandatory. Fields marked with a grey asterisk (*) are mandatory in some circumstances.
Saving your draft application
If you need to exit your application before it is complete, you can save it as a draft.
From within the application screen, click on the Save button at the bottom right of your browser window.

Your changes will be saved as a draft. You can now close your application.
When you next log in to your Business Portal, go to the My work menu and click on the arrow beside Work on drafts.

This will open a list of your drafts. Choose your draft determination/designation application from this list.

- Double-click on the Designation/determination Application to reopen your draft and continue.
The Applicant Details tab
Enter the applicant details on the Applicant Details tab.
The first field on this tab is Applicant name. This is a mandatory field and will be automatically populated based on your login details.

If you are logged is as a sponsor you do not need to complete step 2 or step 4. You must complete step 3.
Agents will need to complete all steps.
The next field is Sponsor organisation. This field is mandatory. Click on the arrow at the right of the field and select the appropriate sponsor organisation from the dropdown list.

Next, select the appropriate Regulatory correspondence address from the drop down menu.

Next, indicate whether you wish us to Send fee invoice to sponsor. Select either the Yes or No radio button as appropriate.

Once you have selected a sponsor, you will be able to choose the appropriate Billing address from the associated dropdown lists.

The next field allows you to select a Primary contact person. Click on the down arrow at the right of the field to select the appropriate contact person from the dropdown list.
The Name, Telephone number and Email fields will auto-populate once you have made your selection.

If you are logged in as a sponsor, the fields above will already be populated.
You can change the auto-populated details by clicking on the Clear button at the right of the Primary contact person field.
Finally, you may also nominate a Secondary contact person. This field is not mandatory.
Click on the down arrow at the right of the field to select from the dropdown list. The Name, Telephone number and Email fields will auto-populate once you have made your selection.

When you are done, click on the Next button at the bottom left of the browser window to move to the next tab.

The Application Scope tab
The Application Scope tab offers explanatory information on the application process and allows you to indicate if you are applying for Priority review determination, Provisional determination and/or Orphan drug designation. You can also upload supporting documentation from this tab.
Select which determination and/or designation you wish to apply for.
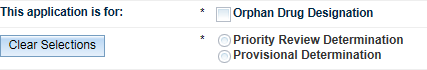
You may select Priority Review Determination OR Provisional Determination AND/OR Orphan Drug Designation.
If you make an incorrect selection, click on the Clear Selections button to clear your selection.

Priority Review Determination
Selecting Priority Review Determination will open explanatory text and a number of related fields:
- New medicine
- Serious condition
- Comparison against existing therapeutic products
- Major therapeutic advance

These fields are mandatory. Select an answer for each field in line with the guidance provided.
Provisional Determination
Selecting Provisional Determination will open explanatory text and a number of related fields:
- New medicine
- Serious condition
- Comparison against existing therapeutic products
- Major therapeutic advance
- Clinical study plan

These fields are mandatory. Select an answer for each field in line with the guidance provided.
Orphan Drug Designation
Selecting Orphan Drug Designation will open explanatory text and a number of related fields.
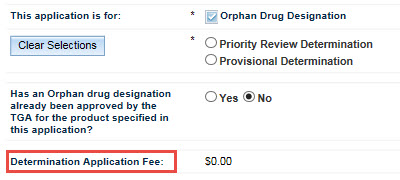
The next field asks you to indicate whether the product has already been designated an Orphan drug for the proposed indication. Select either Yes or No as appropriate.

If you wish to select No, you must first click on Yes and then on No to auto-populate the Determination Application Fee field.
The next field, Determination Application Fee, will be auto-populated. If you select Yes for the previous question, the field will auto-populate with $0.00. If you select No, the field will auto-populate with the appropriate fee.
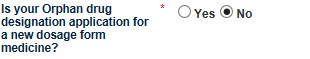
Is your Orphan drug designation application for a new dosage form medicine? Select Yes or No depending on the orphan designation type you are applying for.
A set of different criteria will display depending on whether you selected Yes or No to the above question.
- Indication
- Seriousness
- Medical plausibility
- Prevalence threshold OR lack of financial viability
- Overseas regulators and safety
- Comparison with registered therapeutic goods
Orphan drug designation - Not a New Dosage Form eligibility criteria

OR
Orphan drug designation - New dosage form eligibility criteria

These fields are mandatory. Select an answer for each field in line with the guidance provided.
There are no fees for Orphan drug designation applications.
If you are applying for both Orphan drug designation and either Priority review or Provisional determination at the same time, you will be charged the applicable determination fee. However, if your Orphan drug designation is granted, your determination application fee will be refunded.
The Application Scope tab also offers a link to the Application Checklists, which will become available depending on the application scope you select at the top of the tab.

These links will take you to a form page, where you can access the application checklists under D for Designation checklist.

You can upload Supporting Documentation at the bottom of this tab. See our guidance on the eligibility criteria and supporting documentation for Priority review determination, Provisional determination and Orphan drug designation for more information.
Note: the Add and Remove buttons will be greyed out until you have saved the form at least once. To activate these buttons, click on the Save button at the bottom right of your window.

Once you have saved the document, the Add and Remove buttons will become active. Click on the Add button to upload a supporting document.
This will open an Attachment Details pop-up window.

Both fields in the Attachment Details pop-up window, Description and Supporting Document, are mandatory. Enter a description of the file and then click on the Browse button at the right of the Supporting Document field to find and upload the document.
- Click on Save & Close to upload your file.
Any file you upload must meet the formatting requirements outlined in parts A and B of the general dossier requirements.
Upload separate documents for each determination and designation you are applying for.
Click on the Next button at the bottom left of the browser window to move to the next tab, or click on Previous to go back.

The Product Details tab
Enter information about your product on the Product Details tab.
The first field asks if your medicine is an existing TGA registered product. Select Yes or No as appropriate.

If you select Yes, two additional fields, ARTG Number(s) and Existing indication, will open.
Enter the ARTG Number of your product in the ARTG Number(s) field.

Next, enter the active ingredient in the Name of active ingredient(s) field. This field is mandatory.

This form does not validate the ARTG number and ingredients against the ARTG database. Please enter this information correctly.
Enter the existing indication for your product in the Existing indication field.

In the Proposed indication(s) field, enter the indication you propose the product be used for. This field is also mandatory.

For Orphan designations two additional questions (6 and 7 below) will be displayed.
The next field Is the proposed indication the same as the proposed orphan indication? will only appear if you are applying for Orphan drug designation. Select Yes or No as appropriate.

If you select No another field, Proposed orphan indication, will open.

Enter your proposed orphan indication in this field.
In the Dose form(s) field, select all the forms the product is supplied in. You may select as many dosage forms as apply.
Tick the box to the left of the dose form you wish to select. Use your mouse to scroll through the listed dose forms, or click on the arrows at the right of the field to move up and down through the list.
Enter the trade name of the product in the Trade name field. This field is not mandatory.

The next field, Is the product for prevention, diagnosis or treatment? is mandatory. Select the appropriate answer from the three radio buttons.

Select Yes or No for the next field, Is there a companion diagnostic to this product?. This field is also mandatory.

If you answer Yes to this question, you will not be required to provide more information on the companion diagnostic in the form. However, you should include information on the companion diagnostic in your supporting documentation which is attached to the form.
Requirements are outlined in our guidance on the eligibility criteria and supporting documentation for Priority review, Provisional determination and Orphan drug designation.
If there have been any overseas regulatory submissions for the product, or it has been granted a designation (or equivalent status) by a comparable overseas regulator, enter the details in the next field.

The final field on this tab is also mandatory. Select the Yes or No radio button to indicate whether the product has been refused approval by any overseas regulatory agency for safety or efficacy reasons.

If you select Yes, an additional field will open. Enter the details of the refusal in this field.

Click on the Next button at the bottom left of the browser window to move to the next tab, or click on Previous to go back.

The Administration tab
Enter details about previous applications, clearances and other administrative matters on the Administration tab.
The first field on this tab is Manufacturing site clearances (only if applying for Priority review determination). Select the appropriate radio button to answer this question, either Yes or Lodged. This field will not appear unless you are applying for Priority review determination.

If you have lodged a clearance application with TGA, provide the GMP reference number in the next field. This field is optional if you are applying for Provisional determination and/or Orphan drug designation.

Please include reference numbers for all applications that have been lodged or approved by TGA. If you have selected Lodged in response to the question above, entering information into this field is mandatory.
The next field, Proposed date of submission for registration lodgement to the TGA, is mandatory. Click on the calendar icon at the right of the field to select the appropriate date.

The Nominated TGA clinical unit for this application field is also mandatory. Select a clinical unit from the dropdown list at the right of this field.

If you need more information on clinical units, click on the link to the right of this field.

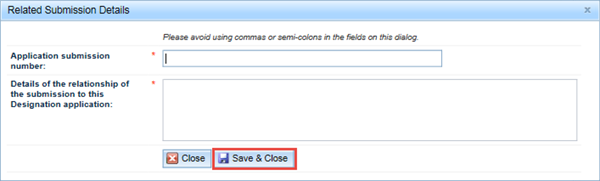
The final fields on this tab allow you to enter information on any other related applications that TGA may currently be evaluating OR relevant lapsed Priority review or Provisional determination applications, or Orphan drug designation applications. Click the Add button to enter details.

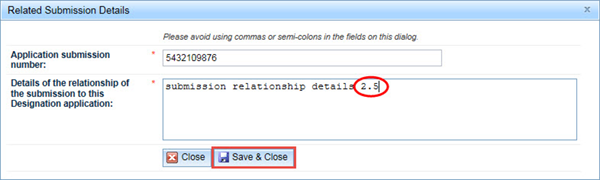
In the Related Submission Details window, enter the Application submission number of any related application, and provide details of how the submission is related to your application in the next field. Click Save & Close.
Please do not use commas or semicolons in this dialog box.
If you wish to add more submissions, repeat these steps until you have added them all.
You can edit a submission by double clicking on the relevant row.

Making the required changes, then clicking Save & Close.

You can remove a submission by ticking the check-box to the left of the submission and clicking the Remove button.

Validating your application
After you have saved the form, the Validate radio button will become active. You must validate your application before you can submit it.
Validating
Click on the Validate button at the bottom right of your browser window.

This will trigger a pop-up window.

- If there are any issues with your application, a new pane will open on the right of your browser window. The issues will be listed in this pane.
If you double-click on a listed validation issue in this pane, it will open your application at the appropriate tab so you can rectify the issue. (This screenshot shows example messages only. You may have no messages or different messages).
When you have rectified all issues, click validate again. Once validation is successful the Submit button at the bottom right of your browser window will become active.

You are now ready to submit your application.
Submitting your application
When you are ready to submit your application, click on the Submit button at the bottom right of the browser window. This will open a Declaration pop-up window.
Declaration
Check the details in the declaration. If the details are correct and you agree with the acknowledgements, click on the Agree button at the bottom left of the pop-up.

Your submission will be lodged and you will be assigned an application number.

You can close the completed application by clicking on the Close button at the bottom right of your browser window.
Application assessment
For more information on how we will assess your application(s), see the Priority review determination, Provisional determination and Orphan drug designation step-by-step guides.
If you have read the user guide and still require assistance, please contact: AET.Application.Entry.Team@health.gov.au.
| Version | Description of change | Author | Effective date |
|---|---|---|---|
| V1.0 | Original publication | Prescription Medicines Authorisation Branch | July 2017 |
| V1.1 | Updates to include the provisional pathway and other minor edits | Prescription Medicines Authorisation Branch and Regulatory Guidance Team | March 2018 |

